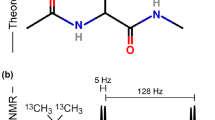
Abstract
Two experiments are presented that yield amino acid type identification of individual residues in a protein by editing the 1H–15N correlations into four different 2D subspectra, each corresponding to a different amino acid type class, and that can be applied to deuterated proteins. One experiment provides information on the amino acid type of the residue preceding the detected amide 1H–15N correlation, while the other gives information on the type of its own residue. Versions for protonated proteins are also presented, and in this case it is possible to classify the residues into six different classes. Both sequential and intraresidue experiments provide highly complementary information, greatly facilitating the assignment of protein resonances. The experiments will also assist in transferring the assignment of a protein to the spectra obtained under different experimental conditions (e.g. temperature, pH, presence of ligands, cofactors, etc.).




Similar content being viewed by others
References
Atreya HS, Chary KVR (2001) Selective ‘unlabeling’ of amino acids in fractionally C-13 labeled proteins: an approach for stereospecific NMR assignments of CH3 groups in Val and Leu residues. J Biomol NMR 19:267–272
Atreya HS, Sahu SC, Chary KVR, Govil G (2000) A tracked approach for automated NMR assignments in proteins (TATAPRO). J Biomol NMR 17:125–136
Barnwal RP, Rout AK, Atreya HS, Chary KVR (2008) Identification of C-terminal neighbors of amino acid residues without an aliphatic 13Cγ as an aid to NMR assignments in proteins. J Biomol NMR 41:191–197
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Dötsch V, Wagner G (1996) Editing for amino-acid type in CBCACONH experiments based on the 13Cβ–13Cγ coupling. J Magn Reson B 111:310–313
Dötsch V, Oswald RE, Wagner G (1996a) Amino-acid-type-selective triple-resonance experiments. J Magn Reson B 110:107–111
Dötsch V, Oswald RE, Wagner G (1996b) Selective identification of threonine, valine, and isoleucine sequential connectivities with a TVI-CBCACONH experiment. J Magn Reson B 110:04–308
Dötsch V, Matsuo H, Wagner G (1996c) Amino-acid-type identification for deuterated proteins with a β-carbon-edited HNCOCACB experiment. J Magn Reson B 112:95–100
Feng W, Rios CB, Montelione GT (1996) Phase labeling of C–H and C–C spin-system topologies: application in PFG-HACANH and PFG-HACA(CO)NH triple-resonance experiments for determining backbone resonance assignments in proteins. J Biomol NMR 8:98–104
Feuerstein S, Plevin MJ, Willbold D, Brutscher B (2012) iHADAMAC: a complementary tool for sequential resonance assignment of globular and highly disordered proteins. J Magn Reson 214:329–334
Grzesiek S, Bax A (1993) Amino acid type determination in the sequential assignment procedure of uniformly 13C/15N-enriched proteins. J Biomol NMR 3:185–204
Johnson BA, Blevins RA (1994) NMRView: a computer program for the visualization and analysis of NMR data. J Biomol NMR 4:603–614
Krishnarjuna B, Jaipuria G, Thakur S, D’Silva P, Atreya HS (2011) Amino acid selective unlabeling for sequence specific resonance assignments in proteins. J Biomol NMR 49:39–51
Lee KM, Androphy EJ, Baleja JD (1995) A novel method for selective isotope labeling of bacterially expressed proteins. J Biomol NMR 5:93–96
Lescop E, Brutscher B (2009) Highly automated protein backbone resonance assignment within a few hours: the BATCH strategy and software package. J Biomol NMR 44:43–57
Lescop E, Rasia R, Brutscher B (2008) Hadamard amino-acid-type edited NMR experiment for fast protein resonance assignment. J Am Chem Soc 130:5014–5015
Löhr F, Pérez C, Köhler R, Rüterjans H, Schmidt JM (2000) Heteronuclear relayed E.COSY revisited: determination of 3 J(Hα, Cγ) couplings in Asx and aromatic residues in proteins. J Biomol NMR 18:13–22
McIntosh LP, Dahlquist FW (1990) Biosynthetic incorporation of 15N and 13C for assignment and interpretation of nuclear magnetic resonance spectra of proteins. Q Rev Biophys 23:1–38
Muchmore DD, McIntosh LP, Russell CB, Anderson DE, Dahlquist FW (1989) Expression and nitrogen-15 labeling of proteins for proton and nitrogen-15 nuclear magnetic resonance. Methods Enzymol 177:44–73
Muhandiram DR, Johnson PE, Yang D, Zhang O, McIntosh LP, Kay LE (1997) Specific 15N, NH correlations for residues in 15N, 13C and fractionally deuterated proteins that immediately follow methyl-containing amino acids. J Biomol NMR 10:283–288
Nietlispach D, Ito Y, Laue E (2002) A novel approach for the sequential backbone assignment of larger proteins: selective intra-HNCA and DQ-HNCA. J Am Chem Soc 124:11199–11207
Ohki S, Kainosho M (2008) Stable isotope labeling methods for protein NMR. Prog Nucl Magn Reson Spectrosc 53:208–226
Pantoja-Uceda D, Santoro J (2008) Amino acid type identification in NMR spectra of proteins via beta- and gamma-carbon edited experiments. J Magn Reson 195:187–195
Rios CB, Feng W, Tashiro M, Shang Z, Montelione GT (1996) Phase labeling of C–H and C–C spin-system topologies: application in constant-time PFG-CBCA(CO)NH experiments for discriminating amino acid spin-system types. J Biomol NMR 8:345–350
Schubert M, Smalla M, Schmieder P, Oschkinat H (1999) MUSIC in triple-resonance experiments: amino acid type-selective H-1-N-15 correlations. J Magn Reson 141:34–43
Schubert M, Oschkinat H, Schmieder P (2001a) MUSIC, selective pulses, and tuned delays: amino acid type-selective H-1-N-15 correlations, II. J Magn Reson 148:61–72
Schubert M, Oschkinat H, Schmieder P (2001b) MUSIC and aromatic residues: amino acid type-selective H-1-N-15 correlations, III. J Magn Reson 153:186–192
Schubert M, Oschkinat H, Schmieder P (2001c) Amino acid type-selective backbone 1H–15 N-correlations for Arg and Lys. J Biomol NMR 20:379–384
Schubert M, Labudde D, Leitner D, Oschkinat H, Schmieder P (2005) A modified strategy for sequence specific assignment of protein NMR spectra based on amino acid type selective experiments. J Biomol NMR 31:115–127
Tong KI, Yamamoto M, Tanaka T (2008) A simple method for amino acid selective isotope labeling of recombinant proteins in E. coli. J Biomol NMR 42:59–67
Tugarinov V, Kay LE (2003) Ile, Leu, and Val methyl assignments of the 723-residue malate synthase G using a new labeling strategy and novel NMR methods. J Am Chem Soc 125:13868–13878
Wittekind M, Müller L (1993) HNCACB a high-sensitivity 3D NMR experiment to correlate amide-proton and nitrogen resonances with the alpha- and beta-carbon resonances in proteins. J Magn Reson B 101:201–205
Yamazaki T, Lee W, Arrowsmith CH, Muhandiram DR, Kay LE (1994) A suite of triple resonance NMR experiments for the backbone assignment of 15N, 13C, 2H labeled proteins with high sensitivity. J Am Chem Soc 116:11655–11666
Acknowledgments
This work was supported by projects CTQ2008-00080 and CTQ2011-22514 from the Spanish Ministerio de Ciencia e Innovación.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pantoja-Uceda, D., Santoro, J. New amino acid residue type identification experiments valid for protonated and deuterated proteins. J Biomol NMR 54, 145–153 (2012). https://doi.org/10.1007/s10858-012-9665-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-012-9665-y