Abstract
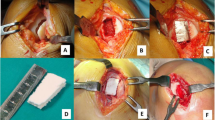
Remedying patellofemoral osteochondral defects using clinical therapy remains challenging. Construct-based and cell-based regenerative medicine with in vitro physical stimuli has been progressively implemented. However, the effect of physical stimuli in situ in knee joints with degradable constructs is still not well-documented. Therefore, we studied whether it was practical to achieve articular cartilage repair using a poly(lactic-co-glycolic acid) (PLGA) construct in addition to early short-term continuous passive motion (CPM) for treatment of full-thickness osteochondral defects in the lower-weigh bearing (LWB) zone of the femoral trocheal groove. Twenty-six rabbits were randomly allocated into either intermittent active motion (IAM) or CPM treatment groups with or without PLGA constructs, termed PLGA construct-implanted (PCI) and empty defect knee models, respectively. Gross observation, histology, inflammatory cells, which were identified using H&E staining, total collagen and alignment, studied qualitatively using Masson’s trichrome staining, glycosaminoglycan (GAG), identified using Alcian blue staining, and newly formed bone, observed using micro-CT, were evaluated at 4 and 12 weeks after surgery. Repair of osteochondral defects in the PCI-CPM group was more promising than all other groups. The better osteochondral defect repair in the PCI-CPM group corresponded to smooth cartilage surfaces, no inflammatory reaction, hyaline cartilaginous tissues composition, sound collagen alignment with positive collagen type II expression, higher GAG content, mature bone regeneration with osteocyte, clear tidemark formation, and better degradation of PLGA. In summary, the use of a simple PLGA construct coupled with passive motion promotes positive healing and may be a promising clinical intervention for osteochondral regeneration in LWB defects.






Similar content being viewed by others
References
Arrigoni, E., S. Lopa, L. de Girolamo, D. Stanco, and A. T. Brini. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: from small to large animal models. Cell Tissue Res. 338:401–411, 2009.
Chang, N. J., Y. R. Jhung, N. Issariyakul, C. K. Yao, and M. L. Yeh. Synergistic stimuli by hydrodynamic pressure and hydrophilic coating on PLGA scaffolds for extracellular matrix synthesis of engineered cartilage. J Biomater. Sci. Polym. Ed., 2011. doi:10.1163/092050611X092611648.
Chang, N. J., C. C. Lin, C. F. Li, N. Issariyakul, and M. L. Yeh. The combined effects of continuous passive motion treatment and acellular PLGA implants on osteochondral regeneration in the rabbit. Biomaterials 33:3153–3163, 2012.
Chu, C. R., M. Szczodry, and S. Bruno. Animal models for cartilage regeneration and repair. Tissue Eng. B Rev. 16:105–115, 2010.
Concaro, S., F. Gustavson, and P. Gatenholm. Bioreactors for tissue engineering of cartilage. Adv. Biochem. Eng. Biotechnol. 112:125–143, 2009.
Du Plessis, M., E. Eksteen, A. Jenneker, E. Kriel, C. Mentoor, T. Stucky, D. van Staden, and L. D. Morris. The effectiveness of continuous passive motion on range of motion, pain and muscle strength following rotator cuff repair: a systematic review. Clin. Rehabil. 25:291–302, 2011.
Ferretti, M., A. Srinivasan, J. Deschner, R. Gassner, F. Baliko, N. Piesco, R. Salter, and S. Agarwal. Anti-inflammatory effects of continuous passive motion on meniscal fibrocartilage. J. Orthop. Res. 23:1165–1171, 2005.
Grigolo, B., G. Lisignoli, G. Desando, C. Cavallo, E. Marconi, M. Tschon, G. Giavaresi, M. Fini, R. Giardino, and A. Facchini. Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng. C 15:647–658, 2009.
Haasper, C., J. Zeichen, R. Meister, C. Krettek, and M. Jagodzinski. Tissue engineering of osteochondral constructs in vitro using bioreactors. Injury 39(Suppl. 1):S66–S76, 2008.
Hinman, R. S., and K. M. Crossley. Patellofemoral joint osteoarthritis: an important subgroup of knee osteoarthritis. Rheumatology (Oxford) 46:1057–1062, 2007.
Howard, J. S., C. G. Mattacola, S. E. Romine, and C. Lattermann. Continuous Passive Motion, Early Weight Bearing, and Active Motion following Knee Articular Cartilage Repair. Cartilage 1:276–286, 2010.
Hunter, D. J., W. Harvey, K. D. Gross, D. Felson, P. McCree, L. Li, K. Hirko, B. Zhang, and K. Bennell. A randomized trial of patellofemoral bracing for treatment of patellofemoral osteoarthritis. Osteoarthritis Cartilage 19:792–800, 2011.
Igarashi, T., N. Iwasaki, D. Kawamura, Y. Kasahara, Y. Tsukuda, N. Ohzawa, M. Ito, Y. Izumisawa, and A. Minami. Repair of articular cartilage defects with a novel injectable in situ forming material in a canine model. J. Biomed. Mater. Res. A 100:180–187, 2012.
Ikeda, R., H. Fujioka, I. Nagura, T. Kokubu, N. Toyokawa, A. Inui, T. Makino, H. Kaneko, M. Doita, and M. Kurosaka. The effect of porosity and mechanical property of a synthetic polymer scaffold on repair of osteochondral defects. Int. Orthop. 33:821–828, 2009.
Im, G. I., H. J. Kim, and J. H. Lee. Chondrogenesis of adipose stem cells in a porous PLGA scaffold impregnated with plasmid DNA containing SOX trio (SOX-5,-6 and -9) genes. Biomaterials 32:4385–4392, 2011.
Jin, C. Z., J. H. Cho, B. H. Choi, L. M. Wang, M. S. Kim, S. R. Park, J. H. Yun, H. J. Oh, and B. H. Min. The maturity of tissue-engineered cartilage in vitro affects the repairability for osteochondral defect. Tissue Eng. A 17:3057–3065, 2011.
Kim, H. K., R. G. Kerr, T. F. Cruz, and R. B. Salter. Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J. Rheumatol. 22:1714–1721, 1995.
Kim, T. K., K. K. Park, S. W. Yoon, S. J. Kim, C. B. Chang, and S. C. Seong. Clinical value of regular passive ROM exercise by a physical therapist after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 17:1152–1158, 2009.
Kocher, M. S., R. Tucker, T. J. Ganley, and J. M. Flynn. Management of osteochondritis dissecans of the knee: current concepts review. Am. J. Sports Med. 34:1181–1191, 2006.
Lu, L., S. J. Peter, M. D. Lyman, H. L. Lai, S. M. Leite, J. A. Tamada, S. Uyama, J. P. Vacanti, R. Langer, and A. G. Mikos. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials 21:1837–1845, 2000.
Mano, J. F., and R. L. Reis. Osteochondral defects: present situation and tissue engineering approaches. J. Tissue Eng. Regen. Med. 1:261–273, 2007.
Martin, I., S. Miot, A. Barbero, M. Jakob, and D. Wendt. Osteochondral tissue engineering. J. Biomech. 40:750–765, 2007.
Martin-Hernandez, C., J. Cebamanos-Celma, A. Molina-Ros, J. J. Ballester-Jimenez, and J. Ballester-Soleda. Regenerated cartilage produced by autogenous periosteal grafts: a histologic and mechanical study in rabbits under the influence of continuous passive motion. Arthroscopy 26:76–83, 2010.
McWalter, E. J., D. J. Hunter, W. F. Harvey, P. McCree, K. A. Hirko, D. T. Felson, and D. R. Wilson. The effect of a patellar brace on three-dimensional patellar kinematics in patients with lateral patellofemoral osteoarthritis. Osteoarthritis Cartilage 19:801–808, 2011.
O’Driscoll, S. W., and N. J. Giori. Continuous passive motion (CPM): Theory and principles of clinical application. J. Rehabil. Res. Dev. 37:179–188, 2000.
O’Driscoll, S. W., F. W. Keeley, and R. B. Salter. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J. Bone Joint Surg. Am. 70:595–606, 1988.
O’Driscoll, S. W., and R. B. Salter. The induction of neochondrogenesis in free intra-articular periosteal autografts under the influence of continuous passive motion. An experimental investigation in the rabbit. J. Bone Joint Surg. Am. 66:1248–1257, 1984.
O’Driscoll, S. W., and R. B. Salter. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin. Orthop. Relat. Res. 208:131–140, 1986.
Oshima, Y., F. L. Harwood, R. D. Coutts, T. Kubo, and D. Amiel. Variation of mesenchymal cells in polylactic acid scaffold in an osteochondral repair model. Tissue Eng. C 15:595–604, 2009.
Pascual-Garrido, C., M. A. Slabaugh, D. R. L’Heureux, N. A. Friel, and B. J. Cole. Recommendations and treatment outcomes for patellofemoral articular cartilage defects with autologous chondrocyte implantation: prospective evaluation at average 4-year follow-up. Am. J. Sports Med. 37:33S–41S, 2009.
Riegger-Krugh, C. L., E. C. McCarty, M. S. Robinson, and D. A. Wegzyn. Autologous chondrocyte implantation: current surgery and rehabilitation. Med. Sci. Sports Exerc. 40:206–214, 2008.
Rosen, J., E. Strauss, A. Schachter, and S. Frenkel. The efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesions. Am. J. Sports Med. 37:720–726, 2009.
Rudert, M. Histological evaluation of osteochondral defects: consideration of animal models with emphasis on the rabbit, experimental setup, follow-up and applied methods. Cells Tissues Organs 171:229–240, 2002.
Salter, R. B. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin. Orthop. Relat. Res. 242:12–25, 1989.
Salter, R. B., D. F. Simmonds, B. W. Malcolm, E. J. Rumble, D. Macmichael, and N. D. Clements. The biological effect of continuous passive motion on the healing of full-thickness defects in articular-cartilage—an experimental investigation in the rabbit. J. Bone Joint Surg. Am. 62:1232–1251, 1980.
Shao, X. X., D. W. Hutmacher, S. T. Ho, J. C. Goh, and E. H. Lee. Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials 27:1071–1080, 2006.
Sun, S., Q. Ren, D. Wang, L. Zhang, S. Wu, and X. T. Sun. Repairing cartilage defects using chondrocyte and osteoblast composites developed using a bioreactor. Chin. Med. J. (Engl.) 124:758–763, 2011.
Sun, Y., Y. Feng, C. Q. Zhang, S. B. Chen, and X. G. Cheng. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int. Orthop. 34:589–597, 2010.
Swieszkowski, W., B. H. Tuan, K. J. Kurzydlowski, and D. W. Hutmacher. Repair and regeneration of osteochondral defects in the articular joints. Biomol. Eng. 24:489–495, 2007.
Taskiran, E., and C. Ozcelik. Autologous osteochondral transplantation. Acta Orthop. Traumatol. Turc. 41:70–78, 2007.
Thermann, H., C. Becher, and A. Driessen. Microfracture technique for the treatment of articular cartilage lesions of the talus. Orthopade. 37:196–203, 2008.
Tok, F., K. Aydemir, F. Peker, I. Safaz, M. A. Taskaynatan, and A. Ozgul. The effects of electrical stimulation combined with continuous passive motion versus isometric exercise on symptoms, functional capacity, quality of life and balance in knee osteoarthritis: randomized clinical trial. Rheumatol. Int. 31:177–181, 2011.
Wang, W., B. Li, J. Yang, L. Xin, Y. Li, H. Yin, Y. Qi, Y. Jiang, H. Ouyang, and C. Gao. The restoration of full-thickness cartilage defects with BMSCs and TGF-beta 1 loaded PLGA/fibrin gel constructs. Biomaterials 31:8964–8973, 2010.
Xie, J., Z. Han, M. Naito, A. Maeyama, S. H. Kim, Y. H. Kim, and T. Matsuda. Articular cartilage tissue engineering based on a mechano-active scaffold made of poly(l-lactide-co-epsilon-caprolactone): in vivo performance in adult rabbits. J. Biomed. Mater. Res. B 94:80–88, 2010.
Zhang, Y., F. Yang, K. Liu, H. Shen, Y. Zhu, W. Zhang, W. Liu, S. Wang, Y. Cao, and G. Zhou. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials, 2012. doi:10.1016/j.biomaterials.2012.1001.1006.
Acknowledgments
This work was supported by the National Science Council of Taiwan, R.O.C. (99-2627-B006-009- and 99-2627-B006-018).
Conflict of Interest
All authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael S. Detamore oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, NJ., Lin, CC., Li, CF. et al. The Effect of Osteochondral Regeneration Using Polymer Constructs and Continuous Passive Motion Therapy in the Lower Weight-Bearing Zone of Femoral Trocheal Groove in Rabbits. Ann Biomed Eng 41, 385–397 (2013). https://doi.org/10.1007/s10439-012-0656-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-012-0656-7