Abstract
Background
Most Chinese blood centers have implemented mini pool (MP) HBV nucleic acid testing (NAT) together with HBsAg ELISA in routine blood donor screening for HBV infection since 2015, and a few centers upgraded MP to individual donation (ID) NAT screening recently, raising urgent need for cost-benefit analysis of different screening strategies. In an effort to prevent transfusion-transmitted infections (TTIs) for HBV, cost-benefit analyses of three different screening strategies: HBsAg alone, HBsAg plus MP NAT and HBsAg plus ID NAT were performed in blood donors from southern China where HBV infection was endemic.
Methods
MP-6 HBV NAT and ID NAT were adopted in parallel to screen blood donors for further comparative analysis. On the basis of screening data and the documented parameters, the number of window period (WP) infection, HBV acute infection, chronic hepatitis B infection (CHB) and occult hepatitis B infection (OBI) was evaluated, and the potential prevented HBV TTIs and benefits of these three strategies were predicted based on cost-benefit analysis by an estimation model.
Results
Of 132,323 donations, the yield rate for HBsAg-/DNA + screened by ID NAT (0.12%) was significantly higher than that by MP NAT (0.058%, P < 0.05). Furthermore, the predicted transfusion-transmitted HBV cases prevented was 1.25 times more by ID NAT compared to MP-6 NAT. The cost-benefit ratio of the universal HBsAg screening, HBsAg plus ID NAT and HBsAg plus MP NAT were 1:58, 1:27 and 1:22, respectively.
Conclusions
Universal HBsAg ELISA screening in combination with HBV ID NAT or MP-6 NAT strategies was highly cost effective in China. To further improve blood safety, HBsAg plus HBV DNA ID NAT screening should be considered in HBV endemic regions/countries.
Similar content being viewed by others
Introduction
HBV infection remains a major global health threat, with 2 billion people worldwide estimated to have a history of past or prior infection and about 5% of the world’s population have chronic hepatitis B virus (HBV) infections (CHBs) [1]. More importantly, more HBV carriers exist and nearly 25% of these carriers will develop into chronic infections potentially leading to cirrhosis, and even hepatocellular carcinoma (HCC) [2,3,4]. In China, hepatitis B infection ranks one of the top three infectious diseases and about 300,000 patients die from HBV-related diseases annually [5]. Additionally, HBV infection is one of the most common contributing factors for cancer deaths [6]. HBV infection is highly epidemic in China, where approximately 50% of the population have a history of HBV infection, and epidemiological studies showed the prevalence of HBsAg reaches as high as 5–10% in general population [7].
In an effort to control hepatitis B infection, Chinese government has been implementing neonatal vaccination program since 1992, which resulted in a significant reduction of HBsAg prevalence in children from 10% to < 1% over the last two decades [8]. To further reduce the residual risk of transfusion-transmitted HBV infection and to improve blood safety, nationwide NAT screening for all the blood donors has been adopted since 2015. Although anti-HBc testing is not routinely performed in Chinese blood centers because deferring anti-HBc-positive units will significantly affect blood supply in HBV medium to high-endemic areas such as in China [9], with the combination of sensitive HBsAg plus NAT screening for blood donors and universal vaccination program in children, the residual risk of transfusion-transmitted HBV infection has been markedly reduced [10, 11]. However, OBIs in blood donors with extremely low viral loads appeared intermittently and are not detectable either by mini pool (MP) NAT or more sensitive individual donation (ID) NAT [12, 13]. Given the higher sensitivity of ID-NAT compared to MP-NAT, ID-NAT has been predominantly employed for detecting OBI donors with extremely low viral loads even though the cost is higher.
Shenzhen Blood Center began to adopt multiplex Mini pool NAT as an option in routine blood screening from 2006, and HBV DNA yield rate was 1:3239 from 307,740 blood donations assayed [10]. ID NAT (Ultrio assay) has been routinely performed in blood donors since 2012, and more low-level viral carriers such as OBIs (OBI rate:1:453) were identified [11]. From 2019, ID NAT and MP NAT were used for screening HBV DNA simultaneously for comparison study. However, a proportion of MP-reactive but ID-nonreactive donations has been detected during routine screening, among which some were OBIs, posing threat of transfusion-transmitted HBV infection [12]. In order to further decrease the residual risk of transfusion-transmitted HBV infection in HBV highly endemic regions such as in China and to adjust the routine screening strategy accordingly, cost-benefit analyses of three different screening strategies: namely HBsAg ELISA alone, HBsAg ELISA plus MP-6 NAT, and HBsAg ELISA plus ID NAT for blood donor screening were conducted in this study by an estimation model. The number of potential transfusion-transmitted HBV cases prevented were predicted, and a more tailored screening strategy was also proposed.
Materials and methods
Routine screening of blood donors
A total of 132,323 donations were screened for HBsAg (ELISA, DiaSorin S.P.A. –UK Branch and WanTai Diagnostics), anti-HCV, anti-HIV, Syphilis and alanine aminotransferase (ALT) from Aug. 2020 to Oct. 2021. HBsAg initial reactive samples were re-tested in duplicate, if results were reactive in any assay as previously reported, and these donations were determined to be HBsAg ELISA+ [12]. All the blood donations were randomly screened by either ID NAT or MP-6 NAT. Multiplex Procleix ultrio plus assay (ID NAT, Grifols diagnostic solutions, Inc. and hogic) and MPX 2.0 (MP-6 NAT, Cobas TaqScreen MPX test, version 2.0, Roche Molecular Systems, Branchburg, NJ) were performed. In total, 66,029 and 66,294 donations were screened by ID NAT and MP-6 NAT respectively for HBV DNA, HCV RNA and HIV-1 RNA. Subsequently, a discriminatory Procleix Ultrio plus HBV/HCV/HIV test (dHBV, dHCV and dHIV) was used to determine Initial reactive samples of ID NAT of Ultrio plus assay for the respective virus (HBV, HCV, or HIV-1). Even if discriminatory Procleix Ultrio plus tests were negative, the initial reactive donations could not be released, and they were labeled as non-discriminators. Meanwhile, MPX–reactive pools (MP-6) were resolved by retesting each individual donation by MPX 2.0 ID NAT, and samples individually reactive were classified as MPX repeat reactive (regarded as NAT+). If one NAT + donation was identified, the other five non-reactive donations were determined as NAT- and can be released. If each individual donation in the mini-pool was detected as MPX nonreactive (MP non-resolved donations), they can be released, and were regarded as HBsAg-/DNA-.
Supplemental serological testing
HBsAg, hepatitis B e antigen (HBeAg), hepatitis B e antibodies (anti-HBe), and hepatitis B core antibodies (anti-HBc) and anti-HBs (LOD: 2 IU/L) were tested by commercial electrochemiluminescence immunoassay (ECLI, Roche, USA) for donations with HBsAg ELISA + and HBV MP NAT + and /or ID NAT + as described in our previous study [9]. HBsAg ELISA + samples were also assayed using the MPX 2.0 ID NAT or the Ultrio Plus ID NAT. Samples that tested positive for HBsAg and HBV DNA with anti-HBc + were considered to have chronic hepatitis B infections (CHBs). Samples that tested positive for HBsAg and HBV DNA without other serological marks were considered to have HBV acute infections (early stage).
Evaluation and calculation of the cost of blood screening tests
The costs of water, electricity, and other instrument usage for HBsAg ELISA accounted for a negligible proportion of the direct reagent cost and were therefore disregarded in this context. The expenses associated with instruments used for HBV NAT screening were provided separately from the reagent costs. To ensure accuracy, we also incorporated Reagent Efficiency (RE) into our analysis. Based on the past two years’ consumption of screening reagents, the RE was calculated as follows.
RE = the number of tests used /the number of specimen reports during Jan. 2020 to Dec. 2021.
Ultro Plus (HBV ID NAT) RE = 1.08; MPX2.0 HBV MP6 NAT RE = 1.31; DiaSorin HBsAg ELISA RE = 1.15; and WanTai HBsAg ELISA RE = 1.15.
The cost of reagent = RE* the total number of specimens tested.
Estimation of residual risk in window period (WP)
A published residual risk (RR) estimation model was adopted to calculate the risk of infection from window period(WP)by ELISA and NAT screening for all blood donors in Shenzhen Blood Center from 2020 to 2021. The RR components for FTDs and RDs were separately calculated. The RR was calculated as the prevalence (p) in donations (per million donations) multiplied by the WP and then divided by the I (Median pre-seroconversion interval) for all serological converters: RR = WP/I * p, and the total donor population risk was added up according to first time donor risk and repeated donor risk as reported previously [14, 15].
Estimation of the risk of transfusion-transmitted hepatitis B infection
The risk factors of WP HBV infection, acute HBV infection, chronic hepatitis B infection and OBI were retrospectively analyzed and estimated as previously described in detail [12, 16, 17].
Estimation of transfusion-transmitted HBV cases prevented
A brief calculation model for predicted transfusion-transmitted HBV cases was established to estimate the total number of transfusion transmitted HBV cases prevented as follows [12].
Number of OBI cases (c): according to previous study [12], we predicted the OBIs cases detected by ID NAT for 132,323 donations: c= [number of OBIs confirmed by ID NAT for 66029 donations+ (ID NAT initial reactive cases- ID NAT yield cases)×46.7%] ×132,323/66,029, and for MP-6 NAT: c = number of OBIs confirmed by MP NAT ×132,323/66,294.
In China, all ID NAT non-confirmed initial reactive donations cannot be released for use, among which 46.7% OBIs were identified based on previous study [18].
Transmission rate of OBI by blood transfusion = 18.2% (11 donor-recipient pairs caused two HBV infections) [16]. Transmission rate of early acute HBV infection by blood transfusion = 100% [19]. Transmission rate of WP HBV infection by blood transfusion = 63% [17]. Transmission rate of CHB by blood transfusion = 40.6% [12].
Predicted transfusion transmitted HBV cases prevented = (a×transmission rate of CHB + b× transmission rate of early acute HBV infection + c×transmission rate of OBI + d×transmission rate of WP HBV infection) × 2 (a donation produces two units washed red blood cells and 200 ml frozen plasma, and at least transfused to two recipients)=(a×40.6%+b×100%+c×18.2%+d×63%)×2.
“a” represented number of CHB cases detected; “b” represented number of acute HBV infections; “c” estimated number of OBI cases detected; “d” represented number of WP infection.
The cases denoted by a, b, c, d are mutually exclusive, and the factor 2 indicating the two ensuing units are independent.
Estimation of the economic benefit of routine blood screening tests
The direct benefit of preventing one case of HBV transmission was calculated as follows:
Benefit (RMB) = Predicted transfusion transmitted HBV cases prevented×420,000, this calculation demonstrated the potential economic benefits associated with intervention, estimated at 420,000 RMB for preventing a single case of HBV infection (1$=6.9RMB) [12].
Estimation of the cost-benefit ratio
The cost-benefit ratio = all the cost of reagents used for screening (ELISA, ELISA + ID NAT or ELISA + MP NAT) not including human power/ benefit.
The specific criteria for determining the cost-effectiveness of the screening strategies is “benefit > cost”, or cost-benefit ratio < 1:1.
Cost-benefit estimation of ID NAT or MP NAT without HBsAg
Additional cost-benefit ratio = (the cost of adding ID NAT or MP NAT- the cost of HBsAg alone)/( the benefit of adding ID NAT or MP NAT- the benefit of HBsAg alone).
Results
Routine screening and additional serological testing results
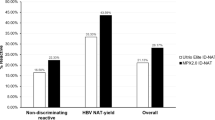
A total of 132,323 donations were screened by HBsAg plus MP-6 NAT or HBsAg plus ID NAT, including 70,789 (53.5%) first time donors and 61,534 (46.5%) repeat donors. 330 donations were HBsAg ELISA + including 111 HBsAg + in a single ELISA test. Among the 132,323, 66,294 donations (11049 MP-6 pools) were tested by MP-6 NAT, and 115 pools were initial reactive, of which 39 donations were resolved HBV DNA+ (0.058%, %95CI: 0.042-0.08%, MP NAT yield). Meanwhile, 66,029 donations were tested by ID NAT, and 186 were initial reactive, among which 77 were determined HBV DNA+ (0.12%, 95%CI: 0.089-0.15%, ID NAT yield). After additional serological and nucleic acid testing, 274 were confirmed as HBV DNA + including 264 first time donors and 10 repeat donors, and 272 were determined HBsAg+ (0.21%, 95%CI:0.18-0.23%) with HBV DNA and anti-HBc, 2 were HBsAg + plus DNA + without other sero-marks. 56 out of 111 HBsAg + samples in a single ELISA test were HBsAg ECLI- and DNA- as determined by various DNA assays and confirmed as HBsAg-. For ID NAT, 3 dHBV + donations were HBsAg ECLI- with DNA- by supplemental DNA assays, including 2 without sero-marks and one with anti-HBs alone, and were regarded as HBsAg - and DNA-. Additionally, there was one donation with HBsAg-/MPX HBV DNA + that was negative for all markers in the supplemental tests. In total, 112 donations (0.084%, 95%CI: 0.07-0.10%) were confirmed OBIs (Fig. 1).
Flowchart for serological and NAT algorithm of 132,323 donations. Classification algorithm of HBV infections was shown. CHB(s), chronic hepatitis B infection(s); ELISA, enzyme-linked immunosorbent assay; HBV, hepatitis B virus; NAT, nucleic acid testing; OBIs, occult hepatitis B infections. dHBV, discriminatory Procleix Ultrio plus HBV test; NRR, non-repeat reactive; MP6, mini pools of six
Estimation of window period residual risks of different screening strategies
The pre-seroconversion interval (days) of 10 repeat donors were documented, and the median (I) was calculated as 738 days. The WPs for HBsAg ELISA alone, HBsAg ELISA plus MP-6 NAT and HBsAg ELISA plus ID NAT were 42, 8.0 and 5.3, respectively [20, 21]. Therefore, the residual risks were estimated as 27.26, 5.19 and 3.44 per 106 donations for HBsAg ELISA alone, HBsAg ELISA plus MP-6 NAT and HBsAg ELISA plus ID NAT, respectively. This would prevent 2.92 (22.07/106 × 132323) and 3.15 (23.82/106 × 132323) HBV infections after adopting MP-6 NAT and ID NAT during the study period correspondingly. The residual risk of window period (WP) infection for the three screening strategies are summarized in Table 1.
Cost-benefits analysis of three screening strategies
After screening 132,323 donations by ELISA, 130,668 seronegative blood samples (21778 pools of MP6) were enrolled in this study for MP-6 NAT. The government bidding purchasing prices of DiaSorin HBsAg ELISA, WanTai HBsAg ELISA, Roche MPX2.0 NAT for HBV DNA (MP-6 format), multiple Procleix Ultrio plus assay for HBV DNA (ID NAT) were 10 RMB/per test, 0.6 RMB/per test, 18 RMB/per test, 23 RMB/per test, respectively. Thus, the cost of each screening strategy was calculated as number of donations * purchasing prices*RE. The screening cost of two or three different reagents consumed were summed up, and the cost-benefit rate of the HBsAg alone, and HBsAg + ID NAT were 1:58.55, 1:27.42 and 1:22.92, respectively (Table 2).
Analysis of the ratio of additional costs divided by additional benefits
Incremental costs / incremental benefits were 3081151.44 (Yuan)/13,139,972(Yuan) for “HBsAg + MP NAT” and 3286903.32(Yuan)/39,933,032(Yuan) for “HBsAg + ID NAT” respectively. Therefore, the ratios of additional cost-benefit were 1:4.26 for “HBsAg + MP NAT” and 1:12.15 for “HBsAg + ID NAT”, respectively.
Example calculation: HBsAg alone
The purchasing price of dual ELISA reagents/per test = DiaSorin HBsAg ELISA + WanTai HBsAg ELISA = 10 RMB/per test + 0.6 RMB/per test = 10.6 (Yuan). RE = 1.15 for ELISA.
Screening cost = number of donations × purchasing prices×RE = 132,323 × 10.6 × 1.15 = 1613017.37 (Yuan).
No OBIs and no WPs can be detected, a = 272, b = 2, c = 0 and d = 0.
So, transfusion transmitted HBV cases prevented by ELISA = (a×40.6%+b×100%) × 2=(272 × 40.6%+2 × 100%) × 2 = 224.864.
Screening Benefit = Predicted transfusion transmitted HBV cases prevented×420,000 = 224.864 × 420,000 = 94,444,288.
Cost-benefit ratio = 1613017.37 ÷ 94,444,288 = 1:58.55.
Example calculation: HBsAg + ID NAT
Screening cost of ID NAT = number of donations × purchasing prices×RE = 132,323 × 23 × 1.08 = 3286903.32 (Yuan).
Screening cost of ID NAT plus HBsAg = 3286903.32 + 1613017.37 = 4899920.69 (Yuan).
c= [number of OBIs confirmed by ID NAT+ (ID NAT initial reactive cases- ID NAT yield cases)×46.7%] × 132,323/66,029=[74+(186 − 77)×46.7%] = 250.31.
So, transfusion transmitted HBV cases prevented by HBsAg + ID NAT = (a×40.6%+b×100%+c×18.2%+d×63%) ×2 =(272 × 40.6%+2 × 100%+250.31 × 18.2%+3.15 × 63%) × 2 = 319.946.
Screening Benefit = Predicted transfusion transmitted HBV cases prevented×420,000 = 319.946 × 420,000 = 134,377,320 (Yuan).
Cost-benefit ratio = 4899920.69 ÷ 134,377,320 = 1:27.42.
Example calculation: HBsAg + MP-6 NAT
Screening cost of MP NAT = number of donations × purchasing prices×RE = 1,306,688 × 18 × 1.31 = 3081151.44 (Yuan).
Screening cost of MP NAT plus HBsAg = 3081151.44 + 1613017.37 = 4694168.81 (Yuan).
c = number of OBIs confirmed by MP NAT× 132,323/66,294 = 38 × 132,323/66,294 = 75.85.
So, transfusion transmitted HBV cases prevented by HBsAg + MP NAT = (a×40.6%+b×100%+c×18.2%+d×63%) ×2 =(272 × 40.6%+2 × 100%+75.85 × 18.2%+2.92 × 63%) × 2 = 256.153.
Screening Benefit = Predicted transfusion transmitted HBV cases prevented×420,000 = 256.153 × 420,000 = 107,584,260 (Yuan).
Cost-benefit ratio = 4694168.81 ÷ 107,584,260 = 1:22.92.
Discussion
Based on previous screening results and the proposed modelling assumptions, the cost-benefit ratios of HBsAg ELISA screening plus additional HBV MP NAT (1:22.92) or ID NAT (1:27.42) were much lower than the critical value of 1:1.0, which fully supported that blood screening strategies for HBV by assaying HBV NAT (ID or MP-6 format) in addition to HBsAg are extremely cost-effective in Chinese blood centers. So far, the international cost-benefit analysis of implementing HBV NAT is based on the Analysis-Markov model prediction method using the quality-adjusted life-years to assess the benefits of life extension and quality improvement, and the outcomes were quite different due to various economic evaluations and different modeling assumptions. A study from Taiwan, China with a high prevalence of HBV reported the cost-effectiveness of adding MP NAT for HBV was much higher than that of performing HBsAg testing. An evaluation in the Netherlands showed that the cost effectiveness of HBV NAT in MP-6 format was better than that of HBV ID NAT [22]. However, in Zimbabwe of South Africa with a high HBV prevalence, data suggested that the introduction of NAT was not cost-effective, even though it could further improve blood safety [23]. However, another study from US concluded that the cost-effectiveness of adding NAT screening is reasonable for ensuring blood safety in comparison with most healthcare interventions [24]. All these data indicated that implementation of NAT screening for blood donors will subsequently result in an increase in the number of prevented viral transmissions and health outcomes gained. Although there is evidence of substantial benefits derived from the introduction of NAT, these benefits come with a significant increase in costs, particularly for blood screening. In current analysis, the cost-benefit ratio of HBV nucleic acid detections (MP-6 or ID NAT) was lower than that of HBsAg detection. The main reason is the high prevalence of hepatitis B infection in China, and most of the blood donors with HBV infections are chronic asymptomatic infections, which can be currently detected by HBsAg ELISA reagents. In addition, the cost of ELISA reagents is much cheaper than NAT screening reagents. Compared to HBsAg ELISA alone, the additional cost-benefit ratio for adding ID NAT stands at 1:12.15, which is 2.85 times as that of adding MP NAT, indicating that the implementation of ID NAT in regions with high HBV prevalence is more cost-effective.
The screening strategy for HBV in blood donors mainly includes HBsAg, HBV NAT and anti-HBc testing worldwide. Undoubtedly, HBsAg plus anti-HBc screening has been proven the most cost-effective due to the high prevalence of hepatitis B infection in China [12]. It is noteworthy that the positive rate of anti-HBc is as high as 40% in Chinese blood donors, and therefore testing anti-HBc would exclude about 40% anti-HBc + donations from blood supply, resulting in blood shortage [7]. Thus, HBsAg and HBV NAT become the mandatory screening strategy in Chinese blood centers. Although the yield rate of HBsAg-/DNA + by ID NAT is higher than MP NAT, the cost of ID NAT seems also too high to afford, therefore MP NAT has logically become a main choice in most Chinese blood centers. As such, it is necessary to analyze the costs and benefits of different screening strategies to provide basic data for government in the decision-making process. During the one year study period, two HBV NAT screening strategies (ID-NAT and MP-NAT) were randomly used, and our results revealed that the yield rate of HBV ID NAT (0.12%) was much higher than that of the MP-6 format (0.058%), mainly because the sensitivity of the HBV ID NAT (95% LOD: 3.4IU/ml) was much higher than that of HBV MP-6 NAT [95% LOD: 13.8 IU/ml [3]. In addition, the estimation of OBI cases detected in ID NAT was 3.30 times higher than in MP-6 format because ID NAT detected many non-discriminators which contained 46.7-69.9% OBIs [18, 25]. Previous study also revealed that HBV MP NAT could result in missing detection of OBIs [8]. An American comprehensive study from 22.4 million blood donors screened by HBsAg, anti-HBc, and NAT also revealed that only 43/404 (10.6%) OBIs could be detected by MP-NAT, while most of OBIs (361/404, 89.4%) with low viral loads could only be identified by ID-NAT [26]. This conclusion was consistent with another previous study which reported HBV MP NAT failed to detect approximately 92% (46 of 593) of OBI donors [27]. Out of 103,356 seronegative Chinese blood donations, Fifty-six out of 98 reactive MPs (57.1%) were resolved as HBV DNA+, but 17 non-resolved donations identified as OBIs by alternative NAT assays were missed by MP-NAT [12]. Transfusion of blood from OBI donations missed by MP NAT may cause HBV infection in recipients. A retrospective study in Italy observed that 2 of 14 patients who received transfusion from OBI donations missed by MP NAT screening were infected, which was confirmed by HBV sequence homology [16]. These evidences strongly suggested ID NAT is needed to detect OBI donors with low viral loads, and it should be given priority to be implemented in routine screening in HBV high endemic regions.
Our current data indicate that serological HBsAg screening in 132,323 blood samples could prevent 224 transfusion-transmitted HBV infection cases. In addition, adding HBV ID NAT or HBV MP-6 NAT screening could intercept 334 and 255 HBV infection cases, respectively. Results of this study also show that HBV ID NAT with HBsAg screening could provide a safer blood, although the cost is higher. The NAT yield rate depends on the assay sensitivity. In our previous studies, we observed an unexpectedly huge increase in NAT yield rates after introduction of the more sensitive Ultrio Plus assay [10, 11]. In addition, the use of MP-6 NAT and/or ID-NAT helps overcome the safety gap resulting from lack of implementation of the anti-HBc testing in Chinese blood donors. It is worth noting that our findings also suggest that the HBsAg testing remained important to ensure blood safety and should be enforced with reagents of higher sensitivity and specificity [28, 29]. Discontinuation of HBsAg donor screening or reliance on ID-NAT alone seems to be unsafe for blood transfusion [29].
Our current study has limitations. This brief analysis depends on data collected from HBV high endemic regions, therefore universal or more appropriate models may be required for generalization. As we mentioned in this study, we used the brief infectivity model which only included the WP, Acute infection, CHB and OBI infection stages. In fact, estimation model including eight HBV infection stages would be more accurate [19]. Although the modification of the HBV LTR (the mean lifetime risk) is designed in this study to compensate for the calculation bias to improve the accuracy of the model, two limitations would influence the precision of the modeling: (1) when dealing with a skewed distribution such as inter-donation intervals, the median pre-seroconversion interval may be a poor approximation of the mean, and (2) an average seroconversion interval is a length biased estimate of an average of all inter-donation intervals. To strengthen the study findings, a standard error of the estimated cost-benefit ratio should be used.
In conclusion, current strategy for HBV screening in Chinese blood donors using HBsAg ELISA and additional ID-NAT or MP-6 NAT is very cost effective. Introducing ID-NAT or MP-NAT, in addition to serological screening, intercepted more donations with potential threat to blood safety despite a significant increase in cost. In regions where economic condition allows, ID NAT should be given priority to further increase the safety of blood transfusion.
Data availability
Availability of data and materials The data for this study is available from the corresponding author on reasonable request.
Abbreviations
- HBV:
-
Hepatitis B virus
- NAT:
-
nucleic acid testing
- MP:
-
mini pool
- ID:
-
individual donation
- WP:
-
window period
- OBI:
-
occult hepatitis B infection
- TTI:
-
transfusion-transmitted infection
- ELISA(s):
-
enzyme-linked immuno-absorbent assay(s)
- ECLI:
-
electrochemiluminescence immunoassay
- LOD:
-
limit of detection
- CHB(s):
-
chronic hepatitis B infection(s)
- HCC:
-
hepatocellular carcinoma
- ALT:
-
alanine aminotransferase
- anti-HBc:
-
antibodies to HBV core
- HBeAg:
-
hepatitis B e antigen
- anti-HBe:
-
hepatitis B e antibodies. RE = Reagent Efficiency (RE)
- RR:
-
residual risk
- dHBV:
-
discriminatory Procleix Ultrio plus HBV test
References
He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK. Major causes of death among men and women in China. N Engl J Med. 2005;353(11):1124–34. https://doi.org/10.1056/NEJMsa050467.
Inoue T, Tanaka Y. Hepatitis B virus and its sexually transmitted infection - an update. Microb Cell. 2016;3(9):420–37. https://doi.org/10.15698/mic2016.09.527.
World Health Organization. Hepatitis B Fact sheet. Updated July 2016. http://www.who.int/mediacentre/factsheets/fs204/en/
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. https://doi.org/10.1016/S0140-6736.
Chinese Center for Disease Control and Prevention. Reports on Infectious Diseases (2005–2016). http://www.chinacdc.cn/tjsj/fdcrbbg/
Islami F, Chen W, Yu XQ, Lortet-Tieulent J, Zheng R, Flanders WD, Xia C, Thun MJ, Gapstur SM, Ezzati M, Jemal A. Cancer deaths and cases attributable to lifestyle factors and infections in China. Ann Oncol. 2017;28:2567–74.
Ye X, Li T, Xu X, Du P, Zeng J, Zhu W, Yang B, Li C, Allain JP. Characterisation and follow-up study of occult hepatitis B virus infection in anti-HBc-positive qualified blood donors in southern China. Blood Transfus. 2017;15(1):6–12. https://doi.org/10.2450/2016.0268-15.
Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, Wang F, Zheng H, Wang F, Guo J, Jia Z, Ma J, Wang H, Luo H, Li L, Jin S, Hadler SC, Wang Y. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–7. https://doi.org/10.1016/j.vaccine.2009.08.048.
Candotti D, Laperche S. Hepatitis B virus blood screening: need for reappraisal of blood safety measures? Front Med. 2018;5:29–34. https://doi.org/10.3389/fmed.2018.00029.
Ye X, Yang B, Zhu W, Zheng X, Du P, Zeng J, Li C. Six-year pilot study on nucleic acid testing for blood donations in China. Transfus Apher Sci. 2013;49(2):318–22. https://doi.org/10.1016/j.transci.2013.08.005.
Ye X, Li T, Li Y, Zeng J, Li R, Xu X. Comparative analysis of hepatitis B virus infections in blood donors born before and after the implementation of universal HBV vaccination in southern China. Transfus Med. 2023;33(1):81–9. https://doi.org/10.1111/tme.12903.
Ye X, Zhao Y, Li R, Li T, Zheng X, Xiong W, Zeng J, Xu M, Chen L. High frequency Occult Hepatitis B Virus infection detected in Non-resolved donations suggests the requirement of Anti-HBc test in blood donors in Southern China. Front Immunol. 2021;28:12:699217. https://doi.org/10.3389/fimmu.2021.699217.
Brojer E, Grabarczyk P, Liszewski G, Mikulska M, Allain JP, Letowska M, et al. Characterization of HBV DNA+/HBsAg- blood donors in Poland identified by triplex NAT. Hepatology. 2006;44(6):1666–74. https://doi.org/10.1002/hep.21413.
Shang G, Seed CR, Wang F, Nie D, Farrugia A. Residual risk of transfusion-transmitted viral infections in Shenzhen, China, 2001 through 2004. Transfusion. 2007;47(3):529–39. https://doi.org/10.1111/j.1537-2995.2006.01146.x.
Spreafico M, Berzuini A, Foglieni B, Candotti D, Raffaele L, Guarnori I, et al. Poor efficacy of nucleic acid testing in identifying occult HBV infection and consequences for safety of blood supply in Italy. J Hepatol. 2015;63(5):1068–76. https://doi.org/10.1016/j.jhep.2015.06.016.
Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency Virus-1, Hepatitis C Virus, and Hepatitis B Virus and Risk of transmission by transfusion. Transfusion. 2009;49(10):2454–89. https://doi.org/10.1111/j.1537-2995.2009.02322.x.
Ye X, Li T, Shao W, et al. Nearly half of Ultrio plus NAT non-discriminated reactive blood donors were identified as occult HBV infection in South China. BMC Infet Dis. 2019;19(1):577–87. https://doi.org/10.1186/s12879-019-4215-9.
Lelie N, Busch M, Kleinman S. Efficacy of different testing scenarios in reducing transfusion-transmitted Hepatitis B Virus (TT-HBV) infection risk. Viruses. 2022;14(10):2263. https://doi.org/10.3390/v14102263.
Grubyte S, Urboniene J, Nedzinskiene L, Jelinskaite A, Zagminas K, Ambrozaitis A, Jancoriene L. Prevalence, incidence and residual risk of transfusion transmitted viruses (HBV, HCV and HIV infections) in Lithuanian blood donors from 2004 to 2018: the incidence/window-period model study. PLoS ONE. 2021;16(2):e0246704. https://doi.org/10.1371/journal.pone.0246704.
GalelSA,SimonTL,WilliamsonPC,AuBuchonJP,WaxmanDA,EricksonY,BertuzisR,DuncanJR,MalhotraK,VaksJ,HuynhN,PateLL.Sensitivity and specificity of a new automated system for the detection of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus nucleic acid in blood and plasma donations.Transfusion.2018;58(3):649–659.https://doi.org/10.1111/trf.14457.
Borkent-Raven BA, Janssen MP, Van DerPoel CL, et al. Cost-effectiveness of additional hepatitis B virus nucleic acid testing of individual donations or minipools of six donations in the Netherlands. Transfusion. 2019;49(2):311–9.
Mafirakureva N, Mapako T, Khoza S, Emmanuel JC, Marowa L, Mvere D, Postma MJ, van Hulst M. Cost effectiveness of adding nucleic acid testing to hepatitis B, hepatitis C, and human immunodeficiency virus screening of blood donations in Zimbabwe. Transfusion. 2016;56(12):3101–11.
Marshall DA, Kleinman SH, Wong JB, AuBuchon JP, Grima DT, Kulin NA, Weinstein MC. Cost-effectiveness of nucleic acid test screening of volunteer blood donations for hepatitis B, hepatitis C and human immunodeficiency virus in the United States. Vox Sang. 2004;86(1):28–40.
Deng X, Guo X, Li T, Laperche S, Zang L, Candotti D. Alternative hepatitis B virus DNA confirmatory algorithm identified occult hepatitis B virus infection in Chinese blood donors with non-discriminatory nucleic acid testing. Blood Transfus. 2022;20(1):8–17.
Dodd RY, Nguyen ML, Krysztof DE, Notari EP, Stramer SL. Blood Donor Testing for Hepatitis B Virus in the United States: is there a case for continuation of Hepatitis B Surface Antigen. Detection? Transfus. 2018;58(10):2166–70.
Ramachandran S, Groves JA, Xia GL, Saá P, Notari EP, Drobeniuc J, Poe A, Khudyakov N, Schillie SF, Murphy TV, Kamili S, Teo CG, Dodd RY, Khudyakov YE, Stramer SL. Recent and occult hepatitis B virus infections among blood donors in the United States. Transfusion. 2019;59(2):601–11.
Ye X, Li T, Li R, Liu H, Zhao J, Zeng J. Molecular characteristics of HBV infection among blood donors tested HBsAg reactive in a single ELISA test in southern China. BMC Infect Dis. 2021;21(1):83–6.
YeX,LiT,ZhangR,LiuH,ZengJ,HongW,LuL,ZhuW,LiS,XuM,WuS,ChenL.Comprehensive analysis of hepatitis B virus infections in blood donors in southern China that are surface antigen positive but nucleic acid testing negative.Transfusion.2020;60(7): 1076-82.https://doi.org/10.1111/trf.15824.
EkiabyME,TanakaJ,vanDrimmelenH,AllainJP,LelieN.Infectivity of hepatitis B virus (HBV) surface antigen (HBsAg) positive plasma with undetectable HBV-DNA: Can HBsAg screening be discontinued in Egyptian blood donors?J Viral Hepat.2022;29(5):330–339.https://doi.org/10.1111/jvh.13666.
Funding
This work was supported by Shenzhen Sanming Project of Medicine (SZSM202311032) and Shenzhen Key Medical Discipline Construction Fund(SZXK070) to XY ; CAMS Initiative for Innovative Medicine (CAMS-2021-I2M-1-060), National key research and development program (2018YFE0107500), Science and Technology Partnership Program (KY201904011), Ministry of Science and Technology of China, Qin Chuangyuan recruited high-level innovation and entrepreneurship talents project of Science and Technology Department of Shaanxi Province (QCYRCXM-2022-56), vertical research project of General Universal Medical Group (UM0223002) to LC; Medical research project of Xi’an Science and Technology Bureau (22YXYJ0120), vertical research project of General Universal Medical Group(UM0223003) to HX and Shengxiang development fund for transfusion medicine of Chinese Society of Blood Transfusion ( CSBT-SX-2022-01) to BL.
Author information
Authors and Affiliations
Contributions
XY, QM and LC designed the experiments and wrote and reviewed the manuscript. BH reviewed, revised, and edited the manuscript. WX collected and analyzed the raw data. XX, JZ, XH BL performed the experiments, and analyzed the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was carried out in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee in Shenzhen Blood Center. Written informed consent was obtained from all subjects participating in this research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Consent to participate
Informed written consent was taken from the participants for this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, X., Xiong, W., Xu, X. et al. Cost-benefit analysis of serological and nucleic acid testing for hepatitis B virus in blood donors in southern China. BMC Infect Dis 24, 909 (2024). https://doi.org/10.1186/s12879-024-09786-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09786-z