Abstract
Background
The prognosis of immunocompromised individuals with COVID-19 remains a significant concern. Information regarding the clinical and virological characteristics of immunocompromised patients infected with SARS-CoV-2 during the Omicron variant period is limited.
Methods
Medical records of patients admitted to our hospital with COVID-19 during the Omicron (BA.1–5) epidemic were retrospectively reviewed. Clinical, virological (nasopharyngeal swabs and blood), and serological data were compared between immunocompromised patients receiving immunosuppressive medications (calcineurin inhibitors, mycophenolate mofetil, or steroids) and control patients not receiving immunosuppressive medications.
Results
Twenty-eight immunocompromised patients (25 transplant recipients) and 26 control patients were included. Fourteen of the immunocompromised patients (50%) received monoclonal antibodies. The immunocompromised group included 15 mild/moderate (53.6%), 10 severe (35.7%), and three critical (10.7%) disease severities. The mortality rate due to COVID-19 during hospitalization was 3.6% (1/28) in the immunocompromised group, with no difference between the two groups. Three cases of re-exacerbation after discharge occurred in the immunocompromised group and none in the control group. Linear regression based on nasopharyngeal real-time-PCR cycle threshold (Ct) values according to the time since symptom onset showed markedly slower viral clearance in the immunocompromised group than in the control group (Pslope = 0.078). In the immunocompromised group, patients who received monoclonal antibodies showed faster viral clearance than those who did not receive monoclonal antibodies. The convalescent anti-spike IgG titers were comparable to those in the control group in patients who received monoclonal antibodies and significantly lower than those in the control patients in patients who did not receive monoclonal antibodies (P < 0.001). The prevalence of viremia at onset was significantly higher in the immunocompromised group than in the control group (35.7%, [10/28] vs. 11.5%, [3/26]; P = 0.003). All three patients with critical disease severity in the immunocompromised group exhibited viremia, one of whom died. All three patients with viremia in the control group were critical, of whom two died.
Conclusions
Immunocompromised individuals receiving immunosuppressive medications are more likely to show delayed post-infection SARS-CoV-2 viral clearance and the development of viremia, potentially resulting in worsening severity and outcomes, especially in viremic patients, even during the Omicron epidemic.
Similar content being viewed by others
Background
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was of concern due to its high mortality rate in immunocompromised patients [1,2,3]. Some studies reported a prolonged period of viral shedding of up to 70 days in immunocompromised patients [4, 5]. Since the end of 2021, Omicron variant lineages have been circulating worldwide. The association of Omicron variant infection with shorter symptom duration, lower severity, and lower hospitalization rates has been reported in the general population [6,7,8]. Regarding immunocompromised patients infected with SARS-CoV-2 after the Omicron wave, the mortality rate in those with hematologic malignancies has been reported to be approximately 8% [9], whereas a few studies on other immunocompromised patients infected with the Omicron variant reported lower mortality rates [10, 11]. However, limited information is available on the clinical and virological characteristics, and prognosis of immunocompromised patients infected with the Omicron variant.
In this study, we collected information on immunocompromised patients with COVID-19 admitted to our hospital after the Omicron wave, which started in January 2022 in Japan. Previous studies on the features of immunocompromised patients with COVID-19 seemed to be unclear regarding the definition of immunocompromised status, and most of the studies focused on patients with various conditions, including cancer and those receiving antitumor or immunosuppressive drugs [9,10,11]. Therefore, in this study, we defined immunocompromised patients as those receiving immunosuppressive medications to clarify their immunocompromised status. These patients are classified as moderately or severely immunocompromised in the US National Institute of Health (NIH) criteria [12]. In addition, we collected data on patients who did not receive immunosuppressive agents and were hospitalized during the same period, as a control group. We collected not only clinical data, such as background, severity, and outcome, but also virological data, including nasopharyngeal and serum viral loads, and serological data on antibody titers. These data were compared between the immunocompromised and control groups.
Methods
Patients
This was a retrospective clinical and virological evaluation of immunocompromised patients with SARS-CoV-2 infection. Patients with COVID-19 admitted to Kyushu University Hospital in Japan from January to October 2022 during the Omicron (BA.1–5) epidemic period [13] were included, and those with at least one nasopharyngeal real-time PCR (RT-PCR) cycle threshold (Ct) value obtained during hospitalization were selected. In this study, patients who had received immunosuppressive medications (calcineurin inhibitors, mycophenolate mofetil, or steroids) at the time of admission and those not receiving immunosuppressive medications were defined as the immunocompromised and control groups, respectively. In the immunocompromised group, the transplant recipients received standard doses of their immunosuppressive drugs that were administered after transplantation, and the remaining patients received NIH-defined steroid doses (≥ 20 mg/day) that potentially satisfied the immunosuppressive status.
Data collection
Patient information and laboratory test results, including nasopharyngeal SARS-CoV-2 RT-PCR Ct values, were collected from medical records. All records and tests were physician dependent. ‘Days after symptom onset’ in this study was based on the number of days from symptom onset at the time of admission, which was recorded by physicians. The severity of COVID-19 was defined based on the US NIH criteria [14]. ‘Mild/moderate’ was defined as SpO2 ≥ 94%, ‘severe’ was defined as SpO2 < 94%, and ‘critical’ was defined as requiring mechanical ventilation.
RT-PCR
Two quantitative RT-PCR methods were used for the detection of SARS-CoV-2 in nasopharyngeal swab specimens: the cobas 6800 system (Roche Molecular Diagnostics, Pleasanton, CA, USA) targeting the E and ORF 1a/b genes, and the GeneXpert system (Cepheid, Sunnyvale, CA, USA) targeting the E and N genes. Samples with a Ct value of 40 or higher were considered negative (no amplification) for SARS-CoV-2 RNA and were treated as a result of a Ct value of 40 [15,16,17].
Qualitative RT-PCR detection of SARS-CoV-2 was performed using serum samples obtained at the time of admission for other biochemical tests. Amplification of the N gene in serum was performed using the amplification conditions described in the Centers for Disease Control and Prevention (CDC) 2019-Novel Coronavirus EUA protocol [18]. Viremia was defined as positive when at least one of the N1, N2, or N3 genes was detected.
Serological testing
Serum samples obtained within 6 days of symptom onset and 7 or more days after symptom onset were used as early and convalescent samples, respectively. Serological testing was performed using the serum samples. The quantitative levels of IgG antibodies for the spike (receptor-binding domain [RBD]) antigen of SARS-CoV-2 were examined using Abbott Architect immunoassays (SARS- CoV-2 IgG II Quant, Abbott, Park, IL, USA) according to the manufacturer’s protocol.
Statistical analysis
Categorical variables were analyzed using the Chi-squared test or Fisher's exact test. Continuous variables were compared using the Wilcoxon signed-rank test. Spearman's rank correlation coefficient and linear regression analysis were used to analyze the correlation between SARS-CoV-2 RT-PCR Ct values and the number of days since symptom onset. For these analyses, one Ct value was randomly selected for each patient when multiple values were obtained. The linear regression analysis used Ct values as the outcome variable and included the following three explanatory variables: the number of days from symptom onset to sample collection, receiving immunosuppressive medications, monoclonal antibody therapy, or viremia; and an interaction term between the number of days from symptom onset and immunocompromised status, monoclonal antibody therapy, or viremia. The slope values were calculated using linear regression analysis. Two- sided P values of < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics and outcomes of immunocompromised patients
The demographics and clinical characteristics of the entire patient cohort are shown in Table 1. Of the 28 patients in the immunocompromised group, 25 (89.2%) were transplant recipients, including 15 kidney, 4 liver, 3 heart, 2 multiorgan, and 1 allogeneic hematopoietic stem cell transplant recipients. In the immunocompromised group, 25 patients (89.2%) received calcineurin inhibitors and steroids, and all immunocompromised patients received multiple immunosuppressive drugs. Dose adjustments for immunosuppressive drugs after COVID-19 were conducted only for mycophenolate mofetil, excluding calcineurin inhibitors and steroids. Of the 18 patients treated with mycophenolate mofetil, 10 (55.6%) and 7 (38.9%) received dose reduction and cessation, respectively. There were no significant differences in the vaccination history between the immunocompromised and control groups. Regarding COVID-19 treatment, 14 patients (50%) in the immunocompromised group and none in the control group received monoclonal antibody therapy (sotrovimab or casirivimab/imdevimab). There were no significant differences in the use of remdesivir between the immunocompromised and control groups (75.0%, [21/28] vs. 57.7% [15/26], P = 0.25).
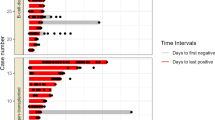
The immunocompromised group included 15 mild/moderate (53.6%), 10 severe (35.7%), 3 critical (10.7%) diseases in the severity of COVID-19. There was no difference in the severity between the immunocompromised and control groups. The mortality rate due to COVID-19 during hospitalization was 3.6% (1/28) in the immunocompromised group, with no significant difference between the two groups. Three patients (10.7%) in the immunocompromised group were readmitted because of symptom exacerbation, with no readmission in the control group (Supplementary Table 1). The Ct values on admission and readmission were 14.3 on day 3 and 20.0 on day12 in Patient 1, 16.6 on day 2 and 24.0 on day20 in Patient 2, and 18.0 on day 1 and 25.0 on day15 in Patient 3. All three patients showed rapid improvement after readmission, with no symptom exacerbation after the second discharge.
Dynamics of SARS-CoV-2 RT-PCR Ct values and anti-spike antibody levels in immunocompromised patients
Changes in SARS-CoV-2 RT-PCR Ct values after symptom onset were compared between the two groups (Fig. 1A). The correlation between the Ct values and the number of days since symptom onset was significant for each group based on the linear regression line (r = 0.705, P < 0.001 in the immunocompromised group; r = 0.765, P < 0.001 in the control group). The slope of the linear regression line was markedly lower in the immunocompromised group than in the control group (0.37 vs, 0.75; interaction (B): − 0.307, 95% confidence interval (CI): − 0.796 to 0.043; P = 0.078), suggesting that the immunocompromised group showed a slower rate of viral clearance in the nasopharynx than the control group. The effects of monoclonal antibody therapy on Ct values were evaluated in the immunocompromised group (Fig. 1B). The slopes in the patients receiving and not receiving antibody therapies were 0.65 and 0.24, respectively (interaction (B): 0.41, 95% CI: − 0.059 to 0.879; P = 0.083).
Comparison of dynamics of nasopharyngeal SARS-CoV-2 RT-PCR Ct values after symptom onset between control and immunocompromised patients (A) and between immunocompromised patients receiving and not receiving monoclonal antibody therapies. The solid line represents the linear regression line for all plots, with the dotted curves indicating the 95% confidence interval (95% CI) for the regression line. The slope value of the linear regression line was calculated using linear regression analysis. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, real-time-PCR; Ct, cycle threshold
The dynamics of anti-spike IgG titers in the immunocompromised and control groups are shown in Supplementary Fig. 1. The median anti-spike IgG titers in immunocompromised patients not receiving monoclonal antibody therapy were significantly lower than those in control patients, both in early serum samples (19.5 vs. 1683.0, P < 0.001) and convalescent serum samples (900.8 vs. 5,0000.0, P < 0.001). The median anti-spike IgG titer in convalescent samples from immunocompromised patients receiving monoclonal antibodies was comparable to that in control patients (2,3000.0 vs. 5,0000.0, P = 0.525).
Clinical and virological characteristics of immunocompromised patients with SARS-CoV-2 viremia
The incidence of SARS-CoV-2 viremia was significantly higher in the immunocompromised group than that in the control group (35.7% [10/28] vs. 11.5% [3/26]; P = 0.003) (Fig. 2). The slope of the linear regression line based on the Ct values after symptom onset in immunocompromised patients with viremia tended to be considerably lower than that in patients without viremia (0.19 vs. 0.65, P = 0.054) (Supplementary Fig. 2). The demographic and clinical characteristics according to the presence or absence of viremia in the immunocompromised group are shown in Table 2. There were no differences in ages, types of organ transplantation, types of immunosuppressive medication, or comorbidities between the two groups. The frequency of booster (third and fourth) vaccinations was significantly higher in the patients without viremia than in those with viremia (50.0% [9/18] vs. 0.0% [0/10], P = 0.0098). All three patients with critical disease in the severity of COVID-19 were included in the viremia group, of whom one died. All three patients with viremia in the control group were critical in terms of severity, of whom two died. The demographic and clinical characteristics of the 13 patients with viremia (10 in the immunocompromised group and 3 in the control group) are shown in Supplementary Table 2.
Discussion
Our study suggested that during the Omicron period of COVID-19, immunocompromised patients showed a slower decline in viral loads than control patients, based on nasopharyngeal SARS-CoV-2 PCR Ct values. This finding differs from that of a previous study with a larger number of immunocompromised patients [16] conducted during the Omicron epidemic. However, the definition of ‘immunocompromised’ used in the previous study included patients with a diverse mix of backgrounds. The immunocompromised group in our study consisted mainly of organ transplant recipients and was limited to patients receiving immunosuppressive medications, which likely explains the difference in the results. Thus, our study suggests that a distinct immunosuppressed status results in slower SARS-CoV-2 clearance in individuals infected with the Omicron variants. Readmission, along with the re-exacerbation of symptoms observed in this study, may reflect delayed viral clearance. Previous reports have suggested virological and/or symptomatic re-exacerbations in immunocompromised patients with COVID-19 [19, 20]. In this study, immunocompromised patients receiving antibody therapy showed a faster reduction in viral load than those not receiving antibody therapy. Anti-spike IgG titers in immunocompromised patients who did not receive antibody therapy barely increased during the convalescent phase. In contrast, during the convalescent phase, anti-spike IgG titers in immunocompromised patients who received antibody therapy increased to levels comparable to those in the control group. These findings suggest that antibody therapy promotes viral clearance. Although half of the immunocompromised patients in our study received antibody therapy, the immunocompromised group in the overall analysis showed markedly slower viral clearance than the control group. In SARS-CoV-2 infected patients receiving immunosuppressive medications, viral clearance is likely to be significantly prolonged during the natural clinical course.
In terms of the comparison between the two groups in this study, a history of immunosuppressive medications did not appear to affect COVID-19 severity; however, the severity of severe and critical diseases accounted for approximately 50% of the immunocompromised group, potentially indicating a higher risk of disease severity. Notably, one-third of immunocompromised patients exhibited SARS-CoV-2 viremia. This likely reflected the depth of immunosuppression. In this study, no immunocompromised patients with viremia received a booster vaccination. It has been reported that SARS-CoV-2 vaccination is associated with protecting against the development of viremia [21]. The anti-spike antibody levels in our immunocompromised group were extremely low at the early onset of symptoms, regardless of the presence of viremia, suggesting that patients receiving immunosuppressive medications could not obtain sufficient antibody responses to SARS-CoV-2 vaccinations. Therefore, we cannot address whether SARS-CoV-2 booster vaccinations contribute to the prevention of viremia in an immunosuppressed state. Further investigation is required to address this issue.
In the overall analysis of this study, most patients with critical severity (6/8) and all patients with an outcome of death (3/3) were accompanied by SARS-CoV-2 viremia. Several studies have reported significant adverse effects and mortality in COVID-19 patients with viremia [22,23,24,25]. These findings suggest that COVID-19 patients, particularly those with immunosuppressive conditions, are likely to be at potential risk of deteriorating the severity of COVID-19, along with viremia. Among the 13 patients with viremia in this study, most patients with critical severity did not receive monoclonal antibody therapy because they were not suitable for treatment at the time of admission. In contrast, most of the remaining patients with mild/moderate and severe severity received antibody therapy. This suggests that COVID-19 patients with viremia may worsen disease severity during the natural clinical course, possibly due to prolonged viral clearance, and that careful observation is necessary during the course of COVID-19, particularly in immunocompromised patients. In addition, this implies the contribution of antibody therapy to viral clearance and the reduction in severity and mortality, as reported previously [10]. After the Omicron variant wave, lower COVID-19 severity was reported in the general population [6,7,8]. However, for patients receiving immunosuppressive medications, categorized as moderately or severely immunocompromised [12], we need to recognize the possibility of a high risk for adverse severity and poor outcomes and the necessity of early treatment from onset, even during the Omicron epidemic.
There are several limitations in this study. The small sample size potentially results in a low statistical power, making it difficult to conclude the COVID-19 clinical course, severity, prognosis, and dynamics of SARS-CoV-2 viral loads in immunocompromised patients. In addition, it was difficult to adjust for potential confounding factors that could influence the outcomes, due to the small sample size. Large scale, multicenter, and international studies are required to resolve this issue. The study was not designed prospectively but was conducted retrospectively based on the physician-dependent collection of clinical information, laboratory data, and serum samples, which affects its reliability. The immunocompromised group in our study focused only on patients receiving immunosuppressive medications; however, this definition might restrict the applicability of the study findings because of the exclusion of patients receiving biological therapies such as rituximab. In this study, only serological immune responses were evaluated. A broader immunological analysis, including T-cell activity and cytokine profiles, should deepen the understanding of the virological and clinical characteristics of immunocompromised patients infected with SARS-CoV-2. In this study, the long-term outcomes of COVID-19 in immunocompromised patients, such as the incidence of persistent symptoms (long COVID), were not evaluated. A longer follow-up period is required in future studies. The control group in this study was not prepared as immunocompetent individuals. Based on the study criteria, patients admitted to our hospital within a certain period were selected as controls. Severity in the control group did not reflect that of COVID-19 in the general population during the Omicron variant epidemic. Since the onset of SARS-CoV-2 pandemic, the Centers for Disease Control and Prevention (CDC) has recommended an isolation period of at least 20 days for moderately to severely immunocompromised patients [26]. Considering our findings, the duration of Omicron variant infectivity in immunocompromised patients treated with immunosuppressive drugs may be longer than that in non-immunocompromised patients. However, in this study, it was difficult to assess the isolation period based on infectivity because viral culture was not performed. The Omicron lineages that have mutated from the BA variants, during of which our study was conducted, have been circulating. Further studies on the relative effects of different Omicron subvariants are warranted.
Conclusions
Despite these limitations, the results of this study are clinically relevant. Even though the control patient was not necessarily immunologically competent, a history of immunosuppressive medications is likely to be associated with the prolongation of SARS-CoV-2 viral clearance and the development of viremia in immunocompromised patients, based on a comparison between the two groups. In addition, the virological features of immunocompromised patients receiving immunosuppressive medications may affect disease severity, symptom remission, and prognosis. Most immunocompromised patients in our study received multiple COVID-19 medications, including antiviral and antibody therapies. Monoclonal antibody therapies against the current Omicron variants are not available because of significant changes in antigenicity. It remains a concern that some immunocompromised patients receiving currently available antiviral medications experience worsening symptoms, severity, and outcomes. Further information needs to be collected on the clinical course, including severity, mortality, and re-exacerbation of immunocompromised patients infected with currently circulating SARS-CoV-2 variants and receiving currently available medications.
Availability of data and materials
The data analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- NIH:
-
National Institute of Health
- RT-PCR:
-
Real-time-Polymerase chain reaction
- Ct:
-
Cycle threshold
- CDC:
-
Centers for Disease Control and Prevention
- RBD:
-
Receptor-binding domain
- CI:
-
Confidence interval
- Ig:
-
Immunoglobulin
References
Hoek RAS, Manintveld OC, Betjes MGH, Hellemons ME, Seghers L, Van Kampen JAA, Caliskan K, van de Wetering J, van den Hoogen M, Metselaar HJ, et al. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int. 2020;33(9):1099–105.
Heldman MR, Kates OS, Safa K, Kotton CN, Georgia SJ, Steinbrink JM, Alexander BD, Hemmersbach-Miller M, Blumberg EA, Multani A, et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic. Am J Transplant. 2022;22(1):279–88.
Kalil AC, Florescu DF. Mortality in solid organ transplant recipients hospitalized for COVID-19. Am J Transplant. 2022;22(1):12–3.
Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, Barbian K, Judson SD, Fischer ER, Martens C, et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020;183(7):1901-1912.e1909.
Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, Dutta J, van Bakel H, Aberg J, García-Sastre A, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med. 2020;383(26):2586–8.
Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, Hinsley W, Bernal JL, Kall M, Bhatt S, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and (B.1.617.2) delta variants in England: a cohort study. Lancet. 2022;399(10332):1303–12.
Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, Louca P, May A, Figueiredo JC, Hu C, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–24.
Christensen PA, Olsen RJ, Long SW, Snehal R, Davis JJ, Ojeda Saavedra M, Reppond K, Shyer MN, Cambric J, Gadd R, et al. Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with Coronavirus Disease 2019 Caused by the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston. Texas Am J Pathol. 2022;192(4):642–52.
Blennow O, Salmanton-García J, Nowak P, Itri F, Van Doesum J, López-García A, Farina F, Jaksic O, Pinczés LI, Bilgin YM, et al. Outcome of infection with omicron SARS-CoV-2 variant in patients with hematological malignancies: An EPICOVIDEHA survey report. Am J Hematol. 2022;97(8):E312–7.
Lafont E, Pere H, Lebeaux D, Cheminet G, Thervet E, Guillemain R, Flahault A. Targeted SARS-CoV-2 treatment is associated with decreased mortality in immunocompromised patients with COVID-19. J Antimicrob Chemother. 2022;77(10):2688–92.
Malahe SRK, Hoek RAS, Dalm VASH, Broers AEC, den Hoed CM, Manintveld OC, Baan CC, van Deuzen CM, Papageorgiou G, Bax HI, et al. Clinical Characteristics and Outcomes of Immunocompromised Patients With Coronavirus Disease 2019 Caused by the Omicron Variant: A Prospective. Observational Study Clin Infect Dis. 2023;76(3):e172–8.
National Institute of Health. COVID-19 Treatment Guidelines. Special Considerations in People Who Are Immunocompromised. https://www.covid19treatmentguidelines.nih.gov/special-populations/immunocompromised/. Accessed 12 Mar 2024.
Nakakubo S, Kishida N, Okuda K, Kamada K, Iwama M, Suzuki M, Yokota I, Ito YM, Nasuhara Y, Boucher RC, et al. Associations of COVID-19 symptoms with omicron subvariants BA.2 and BA.5, host status, and clinical outcomes in Japan: a registry-based observational study. Lancet Infect Dis. 2023;23(11):1244–56.
National Institute of Health. COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed 12 Mar 2024.
Takahashi K, Ishikane M, Ujiie M, Iwamoto N, Okumura N, Sato T, Nagashima M, Moriya A, Suzuki M, Hojo M, et al. Duration of Infectious Virus Shedding by SARS-CoV-2 Omicron Variant-Infected Vaccinees. Emerg Infect Dis. 2022;28(5):998–1001.
Dimcheff DE, Blair CN, Zhu Y, Chappell JD, Gaglani M, McNeal T, Ghamande S, Steingrub JS, Shapiro NI, Duggal A, et al. Total and Subgenomic RNA Viral Load in Patients Infected With SARS-CoV-2 Alpha, Delta, and Omicron Variants. J Infect Dis. 2023;228(3):235–44.
Wong CKH, Lau KTK, Au ICH, Lau EHY, Poon LLM, Hung IFN, Cowling BJ, Leung GM. Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study. Lancet Infect Dis. 2023;23(6):683–95.
Lu X, Wang L, Sakthivel SK, Whitaker B, Murray J, Kamili S, Lynch B, Malapati L, Burke SA, Harcourt J, et al. US CDC Real-Time Reverse Transcription PCR Panel for Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020;26(8):1654–65.
Wong CKH, Lau KTK, Au ICH, Lau EHY, Poon LLM, Hung IFN, Cowling BJ, Leung GM. Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study. Lancet Infect Dis. 2023;23(6):683–95.
Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R: COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022. medRxiv [Preprint]. 2022.
Saá P, Fink RV, Dawar H, Di Germanio C, Montalvo L, Wright DJ, Krysztof DE, Kleinman SH, Nester T, Kessler DA, et al. Prevalence of SARS-CoV-2 Viremia in Presymptomatic Blood Donors in the Delta and Omicron Variant Eras. Open Forum Infect Dis. 2023;10(5):ofad253.
Li Y, Schneider AM, Mehta A, Sade-Feldman M, Kays KR, Gentili M, Charland NC, Gonye AL, Gushterova I, Khanna HK, et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J Clin Invest. 2021;131(13):e148635.
Hagman K, Hedenstierna M, Gille-Johnson P, Hammas B, Grabbe M, Dillner J, Ursing J. Severe Acute Respiratory Syndrome Coronavirus 2 RNA in Serum as Predictor of Severe Outcome in Coronavirus Disease 2019: A Retrospective Cohort Study. Clin Infect Dis. 2021;73(9):e2995–3001.
Ram-Mohan N, Kim D, Zudock EJ, Hashemi MM, Tjandra KC, Rogers AJ, Blish CA, Nadeau KC, Newberry JA, Quinn JV, et al. SARS-CoV-2 RNAemia Predicts Clinical Deterioration and Extrapulmonary Complications from COVID-19. Clin Infect Dis. 2022;74(2):218–26.
Bermejo-Martin JF, García-Mateo N, Motos A, Resino S, Tamayo L, Ryan Murua P, Bustamante-Munguira E, Gallego Curto E, Úbeda-Iglesias A, de la Torre MDC, et al. Effect of viral storm in patients admitted to intensive care units with severe COVID-19 in Spain: a multicentre, prospective, cohort study. Lancet Microbe. 2023;4(6):e431–41.
Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed 31 Mar 2024.
Acknowledgements
We would like to thank Yoshitaka Eto for his technical support.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
KN and YC collected epidemiological data. TG, KS, AY and YE collected serological data. KN, TG and YC performed data analysis and interpretation. KN and YC drafted the manuscript, and YC, KA, and NS revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The requirement for patient consent was waived because the study used de-identified data. The study was approved by the Ethics Committee of Kyushu University (Approval No: 22241–00).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nakamura, K., Goto, T., Shiraishi, K. et al. Clinical and virological features of SARS-CoV-2 Omicron variant-infected immunocompromised patients receiving immunosuppressive medications. BMC Infect Dis 24, 736 (2024). https://doi.org/10.1186/s12879-024-09633-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09633-1