Abstract
Background
Group B Streptococcus (GBS) causes invasive infections in newborns and elderly individuals, but is a noninvasive commensal bacterium in most immunocompetent people. Recently, the incidence of invasive GBS infections has increased worldwide, and there is growing interest in the molecular genetic characteristics of invasive GBS strains. Vaccines against GBS are expected in the near future. Here, we aimed to analyze the molecular epidemiology of GBS according to the invasiveness in South Korea.
Methods
We analyzed GBS isolates collected and stored in two hospitals in South Korea between January 2015 and December 2020. The invasiveness of these isolates was determined via a retrospective review of clinical episodes. Totally, 120 GBS isolates from 55 children and 65 adults were analyzed. Serotype and sequence type (ST) were determined using multiplex polymerase chain reaction (PCR) and multilocus sequence typing, respectively. Fourteen virulence factor-encoding genes of GBS were analyzed using multiplex PCR.
Results
Forty one (34.2%) were invasive infection-related GBS isolates (iGBS). The most frequently detected serotype was III (39/120, 32.5%), and it accounted for a high proportion of iGBS (21/41, 51.2%). The most frequent ST was ST19 (18/120, 15.0%), followed by ST2 (17/120, 14.2%). Serotype III/ST17 was predominant in iGBS (12/41, 29.3%), and all 17 ST2 strains were noninvasive. The distribution of most of the investigated virulence factors was not significantly related to invasiveness; noteworthily, most of the serotype III/ST17 iGBS carried pilus island (PI) 2b (10/12, 83.3%), and the prevalence of fbsB was significantly low compared with noninvasive GBS isolates (P = 0.004). Characteristically, the combination of bca(+)-cspA(+)-pavA(+)-fbsB(-)-rib(+)-bac(-) was predominant in iGBS (24.4%, 10/41).
Conclusions
Serotype III/ST17 GBS carrying PI-2b was frequently detected in iGBS. There was no significant association between invasiveness and the pattern of virulence factors; however, a specific combination of virulence factors was predominant in iGBS.
Similar content being viewed by others
Background
Streptococcus agalactiae (group B streptococcus, GBS) is the leading cause of invasive infections in neonates [1] and opportunistic infections in adults [2]. Invasive diseases associated with GBS infection include bacteremia, meningitis, endocarditis, osteoarticular infections, and pneumonia. In South Korea, GBS is the most common bacterial pathogen causing invasive infections in infants aged less than 3 months [3]. In addition to invasive infections, GBS can colonize the genitourinary and gastrointestinal tracts of healthy adults and pregnant women under non-invasive conditions [4, 5].
The estimated global incidence of neonatal GBS infection including GBS associated preterm birth and stillbirth is 0.49 per 1000 individuals [6]. Recently, the incidence of GBS infections has increased in older adults worldwide [7, 8]. Furthermore, a marked increase in the incidence of GBS infections has been reported in elderly patients with diabetes mellitus and cancer, and those undergoing renal dialysis [9]. The reason of the increase in these cases in not fully understood. Some experts suggested that this increase may be associated with the aging population or changing characteristics of GBS such as serotypes [10, 11]. Several preventive efforts such as GBS screening in pregnancy and intrapartum antibiotic prophylaxis have been adopted. These strategies have markedly decreased early-onset GBS (EOGBS) infections in neonates; however, they have negligible effect on late-onset GBS (LOGBS) infections. In this context, administration of vaccines against GBS is considered an effective preventive method. Although vaccines against GBS have not yet been commercialized, several polysaccharide capsule-based vaccines for pregnant women are expected in the near future [12]. Classically, GBS was classified into 10 serotypes by surface capsular polysaccharides, and that have been studied for the main target of GBS vaccines. Molecular genetic research on GBS has proposed various virulence factors such as pilus island (PI) alleles and surface alpha-like proteins as targets of GBS vaccines [13].
Considering the gradually increasing trend of invasive infections and the development of vaccines against GBS, it is important to monitor the changes in molecular genetic characteristics of GBS. In this study, we aimed to analyze the molecular epidemiology of GBS in South Korea according to the invasiveness, focusing on virulence factors.
Methods
Study population and specimens
From January 2015 to December 2020, a total of 120 GBS isolates were analyzed. The isolates were collected from Seoul National University Bundang Hospital (30 isolates) and Jeju National University Hospital (90 isolates) in Korea and stored at -70 °C via a hospital-wide surveillance system. The samples were obtained from 55 (45.8%) children and 65 (54.2%) adults. GBS strains were isolated from blood (n = 29), cerebrospinal fluid (n = 11), tracheal aspirate (n = 8), urine (n = 43), vaginal discharge (n = 24), and wound samples (n = 4). We determined the invasiveness of GBS through a retrospective medical chart review. Invasive infection-related GBS isolates (iGBS) were identified based on sterile isolation sites and proper invasive clinical episodes.
Analysis of GBS isolates
GBS isolates were identified, and antimicrobial susceptibility tests were performed using an automated microbiology system, Vitek II ID-GPC (bioMérieux, Durham, NC, USA). Automated susceptibility test results were obtained for penicillin, clindamycin, erythromycin, and ciprofloxacin. DNA from GBS isolates was purified using the Solg™ Genomic DNA Prep Kit (Solgent, Daejeon, South Korea), according to the manufacturer’s protocol. GBS serotypes were determined using modified multiplex PCR assays based on the analysis of unique band patterns [14]. Total 20µL PCR mixture using 1.6µL of template DNA with primers [14]. The samples were amplified by a denaturation step for 5 min at 95℃, followed by 25 cycles of 95℃ for 30s, 53℃ for 30s, and 72 for 1 min and a final cycle of 72℃ for 10 min. The PCR products were analyzed by electrophoresis in 1.5% agarose gel. Multilocus sequence typing (MLST) was performed by sequencing seven housekeeping genes (adh, pheS, atr, glnA, sdhA, glcK, and tkt) using Sanger sequencing. Sequencing reactions were performed in the DNA Engine Tetrad 2 Peltier Thermal Cycler (Bio-Rad, Hercules, CA, USA) using the ABI BigDye® Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, Waltham, MA, USA) in Macrogen Corporation (Seoul, Korea). Specific primers for these genes were available in Streptococcus agalactiae MLST database (https://pubmlst.org/sagalactiae). And number of matched alleles of each of the seven housekeeping genes was used to determine the sequence type (ST). The STs were clustered into a clonal complex (CC) using the goeBURST PHYLOViZ program (https://phyloviz.readthedocs.io/en/latest/index.html), and phylogenetic relationships were represented in a diagram according to the invasiveness at single locus variant levels.
After screening researches focusing virulence factor-encoding genes of GBS, the following 14 genes were selected and analyzed: fbsA, fbsB, psvA, lmp, scpB, bac, bca, cfb, cspA, cylE, hylB, rib, pbp1A/ponA, and pilus-related genes. We divided these genes into four sets for multiplex PCR according to base pair size (Table 1). The amplification conditions were similar to those of a previous study [15], with some modifications such as changes of reaction cycles or temperature. A reaction mixture of total volume 20 µL was prepared by mixing 10 µL of 2× Taq Master Mix, 1 µL of template DNA, 0.5 µL of each primer, and 8 µL of distilled water. Initial denaturation at 95 °C for 5 min was followed by 38 cycles of amplification at 94 °C for 30 s, annealing at speci fic temperatures (47 °C for set A, 45 °C for set B, 47 °C for set C, and 52 °C for set D) for 30 s, and a final extension step at 72 °C for 30 s (1 min for set D). The amplified PCR products were visualized on 1.5% agarose gels. The phylogenetic tree was represented using UPGMA algorithm and the distance was expressed as Hamming distance. This tree was combined with the invasiveness and the combination of 6 virulence factors which were detected less than 95.0% of GBS isolates.
Statistical analyses
All data analyses were performed using software R version 3.6.2. (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables are expressed as percentage; they were analyzed using the Chi-square test. Continuous variables are expressed as mean ± standard deviation. Results with P < 0.05 were considered statistically significant.
Results
In this study, the mean age (± standard deviation) of the participants who had GBS isolates was 31.12 ± 32.24 years. Most GBS isolates were obtained from children, pregnant women in their 30s, and elderly individuals in their 80s (Table 2). Among the 120 GBS isolates, 41 (34.2%) were classified to be iGBS, which mostly infected children (37/41, 90.2%). The mean age of the 37 children who had iGBS infection was 0.25 ± 0.72 years, and 5 (13.5%) of them were categorized as having EOGBS infections.
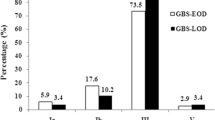
All 120 GBS isolates were susceptible to penicillin. The resistance rates were 45.0% (54/120) for clindamycin, 49.6% (58/117) for erythromycin, and 29.3% (34/116) for ciprofloxacin, relatively. The difference in antibiotic susceptibility according to invasiveness was not significant. Among all isolates, the most frequent capsular serotypes were III (39/120, 32.5%), V (20/120, 16.7%), and VIII (20/120, 16.7%) (Table 3). Serotype III accounted for the highest proportion of iGBS (21/41, 51.2%), and the majority of GBS strains causing meningitis were serotype III (8/11, 72.7%). The proportions of serotypes Ia and IV were as low as 5.8% (7/120) and 3.3% (4/120), respectively. However, these two serotypes mainly caused invasive infections, with serotype Ia accounting for 71.4% (5/7) and serotype IV for 100% (4/4). All of the 20 serotype VIII isolates were noninvasive.
.
In total, 19 STs were identified and grouped into seven CCs. Distributions of STs according to serotypes were shown in Table 4. The most common STs were ST19 (18/120, 15.0%), ST2 (17/120, 14.2%), ST17 (16/120, 13.3%), and ST10 (14/120, 11.7%). The phylogenetic relationships and the proportion of iGBS of each ST are illustrated in Fig. 1. All ST2 (n = 17) isolates were noninvasive and most ST19 isolates (15/18, 83.3%) were noninvasive. Among iGBS, ST17 was the most common (12/41, 29.3%), followed by ST10 (6/18, 14.6%). All ST17 (n = 12) iGBS strains were serotype III and all ST24 (n = 3) iGBS strains were serotype Ia (Table 5).
Phylogenetic diagram of GBS isolates according to invasiveness. Phylogenetic relationships were identified using goeBURST analyses at single locus variant levels. Numbers represent sequence types (STs) based on the multilocus sequence typing (MLST) analysis. Invasiveness is represented by color: red, noninvasive GBS isolates; blue, invasive GBS isolates
The four patterns of PIs and their distribution according to serotype and invasiveness are shown in Table 6. All 120 GBS isolates harbored either PI-2a (70.8%, 85/120) or PI-2b (29.2%, 35/120), and PI-1 was detected in 58.3% of isolates (70/120). The combination of PI-1 and PI-2a was the most frequently detected (53/120, 44.2%). The GBS isolates carrying PI-1 (regardless of the presence of PI-2) were more prevalent in the nGBS group (P = 0.034). Among iGBS carrying PI-2b (regardless of PI-1 status), 90.9% (10/11) were serotype III/ST17. A combination of PI-1 and PI-2b was detected in nGBS (88.2%, 15/17) and serotype VIII (82.4%, 14/17). The pattern of PIs was not related to the isolated sites.
Five virulence factor-encoding genes (cylE, hylB, lmb, scpB, and fbsA) were detected in all 120 GBS isolates. The distribution of other virulence factor-encoding genes was as follows: cfb (119/120, 99.2%), pbp1A/ponA (118/120, 98.3%), bca (112/120, 93.3%), cspA (98/120, 81.7%), pavA (97/120, 80.8%), fbsB (78/120, 65.0%), rib (61/120, 50.8%), and bac (26/120, 21.7%) (Fig. 2). The prevalence of fbsB was significantly lower (P = 0.004) in iGBS compared with nGBS. And the prevalence of rib was relatively high in iGBS (58.5% compared with 46.8% in nGBS), however the difference was not significant (P = 0.306). The following combination of six virulence factors, excluding those commonly present in both iGBS and nGBS, was predominant in iGBS (10/41): the bca(+)-cspA(+)-pavA(+)-fbsB(-)-rib(+)-bac(-) (Fig. 3). Characteristically, all three ST24 isolates caused invasive infections and had the same virulence factor patterns as described above.
Phylogenetic tree of GBS isolates according to the combination of virulence factors. Among 13 virulence factors, 6 virulence factors whose detection rate was less than 95.0% were selected to represent only the significant factors. Combinations of virulence factors with two or fewer isolates were omitted and represented as blank columns. The tree was constructed using the UPGMA algorithm and the distance is expressed as Hamming distance. The red area indicates the proportion of nGBS and the blue area indicates that of iGBS. UPGMA, unweighted pair group method with arithmetic mean; iGBS, invasive GBS isolates; nGBS, noninvasive GBS isolates
Discussion
This study revealed several specific molecular characteristics of iGBS in South Korea. Among iGBS, serotype III was the most common (51.2%), and all of the 20 serotype VIII isolates were nGBS. Among the three prevalent STs (ST19, ST2, and ST17), most ST19 (83.3%) and ST2 (100%) isolates were nGBS. Serotype III/ST17 was the main iGBS strain, and it was found to have PI-2b regardless of PI-1 status (10/12, 83.3%). There were no significant differences of the distribution of the analyzed virulence factor-encoding genes according to invasiveness; however, a specific combination of virulence factor-encoding genes was predominant in iGBS.
Maternal GBS colonization is a major determinant of EOGBS infections in neonates. European studies have shown similarity in serotype distribution between maternal colonized GBS and iGBS, and this finding indicates that EOGBS infection is caused by GBS transmission from mother to newborn [16, 17]. In this study, serotype VIII (8/24, 33.3%) and serotype III (7/24, 29.2%) were the main serotypes of GBS isolated from vaginal discharge. The recent serologic data of GBS among pregnant South Korean women showed that the most common serotypes were V (22.7%) and VIII (20.0%) [18, 19]. However, globally, the frequently distributed serotypes of maternal colonized GBS are I (Ia/Ib), III, and V [5]. In South Korea, the incidence of LOGBS was relatively high compared to the global average [20, 21]. A similar trend of a higher incidence of LOGBS infection was observed in the present study (LOGBS/EOGBS = 5.4). The relatively low prevalence of EOGBS infection in South Korea may be attributed to the high incidence of serotype VIII in isolates from vaginal discharge, which is not highly associated with iGBS infection. This epidemiology is not fully understood, and further study for serotype VIII GBS is needed to be verified.
In this study, the distribution of serotypes and STs between the iGBS and nGBS groups differed significantly. The serotype III/ST17 strain was the most prevalent in iGBS, whereas the serotype VIII/ST2 strain was the most common in nGBS. These results are similar to the distribution of iGBS serotypes with predominant serotype III followed by Ia, Ib, II, and V in other countries [16, 22].
Pili are cell-wall-anchored appendages on GBS cell surface; they play an important role in bacterial attachment to epithelial cells. Three pilus types have been identified in GBS, and all GBS strains have the at least one PI variant [23]. The most frequently detected PI combination is PI-1 and PI-2a [24,25,26], consistent with the results of this study. Hypervirulent ST17 GBS isolates are known to contain the combination of PI-1 and PI-2b, and this combination is rare among other STs [27]. The role of PI-2b in mediating the interaction between GBS and host cell is well known, especially in ST17 GBS [28]. We found a distinctive pattern where PI-2b without PI-1 was dominant in serotype III iGBS. Additionally, several PI-1 and PI-2b combinations were identified in serotype VIII nGBS (Table 6).
The laminin-binding protein encoded by lmb mediates the attachment of GBS to human laminin, and this attachment is crucial for colonization [29]. Fibrinogen-binding protein A (encoded by fbsA) and C5a peptidase (encoded by scpB) help bacteria bind to epithelial cells and fibronectin, respectively. Almost all GBS isolates from humans harbor these virulence factors, which were detected in all GBS isolates in this study. Fibrinogen-binding protein B (fbsB), along with fbsA, was detected in CC17 GBS strains, and the high fibrinogen-binding ability may contribute the invasiveness of GBS in neonates [30]. However, fbsB was rather less frequently detected in iGBS (Fig. 2) and fbsB was mostly detected in non-CC17 strains in this study. Although research on various components of GBS is limited, rib proteins (encoded by rib) and C proteins (encoded by bac and bca) have been considered as vaccine candidates, in addition to capsular polysaccharides and pili [31, 32]. This study revealed differences in the prevalence of these three virulence factors between the iGBS and nGBS groups, with various combinations exhibiting no specific trends. However, the combination of bca(+)-cspA(+)-pavA(+)-fbsB(-)-rib(+)-bac(-) was predominant in the iGBS group. Notably, all ST24 serotype Ia iGBS strains exhibited identical virulence patterns. A Spanish study from the early 2000s reported that the ST24/bca sublineage of serotype Ia might emerge as an important cause of neonatal invasive infections, despite its limited prevalence [33]. This strain may have maintained consistent genotypic pattern unaffected by geographical and temporal variations.
This study had several limitations. Despite collecting isolates over five years from the two hospitals, the number of GBS was relatively small. The hospital surveillance systems were passive surveillance systems, and there were fewer isolates in the early stage, leading to a lack of consistency. Furthermore, the majority of the invasive strains were from children, and the number of iGBS in adults was limited. GBS strains have multiple virulence factors, and not all were included in the study. However, analyzing the molecular characteristics of GBS through a comprehensive comparison between iGBS and nGBS offers valuable insights, and to the best of our knowledge, this is the first study to focus on pili and various virulence factors of GBS in South Korea.
Conclusions
The distribution of serotypes and STs according to invasiveness determined in the present study was similar to that in previous research. The distribution of the PI-1 and PI-2b combination according to serotype was unique, and a specific combination of virulence factors was found. The technical development of several capsular polysaccharide-based GBS vaccines has been completed, and GBS vaccines may soon be commercialized in South Korea. In this milieu, monitoring GBS molecular characteristics in various populations and isolates before and after vaccine implementation is crucial.
Data availability
The dataset used in this study are original. The dataset analyzed during the current study are available from corresponding author on reasonable request.
Abbreviations
- CSF:
-
Cerebral spinal fluid
- CC:
-
Clonal complex
- EOGBS:
-
Early-onset group B Streptococcus
- GBS:
-
Group B infection-related Streptococcus
- iGBS:
-
Invasive group B Streptococcus isolate
- LOGBS:
-
Late-onset group B Streptococcus
- MLST:
-
Multilocus sequence typing
- nGBS:
-
Noninvasive infection-related GBS isolates
- NT:
-
Nontypable serotype
- PI:
-
Pilus island
- PCR:
-
Polymerase chain reaction
- ST:
-
Sequence type
References
Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, Heath PT. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012;379(9815):547–56.
Raabe VN, Shane AL. Group B Streptococcus (Streptococcus agalactiae). Microbiol Spectr 2019, 7(2).
Song SH, Lee HJ, Song ES, Ahn JG, Park SE, Lee T, Cho HK, Lee J, Kim YJ, Jo DS, et al. Changes in etiology of invasive bacterial infections in infants under 3 months of age in Korea, 2006–2020. Pediatr Infect Dis J. 2022;41(12):941–6.
Bliss SJ, Manning SD, Tallman P, Baker CJ, Pearlman MD, Marrs CF, Foxman B. Group B Streptococcus colonization in male and nonpregnant female university students: a cross-sectional prevalence study. Clin Infect Dis. 2002;34(2):184–90.
Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, Lawn JE, Baker CJ, Bartlett L, Cutland C, et al. Maternal colonization with Group B Streptococcus and serotype distribution Worldwide: systematic review and Meta-analyses. Clin Infect Dis. 2017;65(suppl2):S100–11.
Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, Madrid L, Blencowe H, Cousens S, Baker CJ, et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for pregnant women, Stillbirths, and children. Clin Infect Dis. 2017;65(suppl2):S200–19.
Ballard MS, Schønheyder HC, Knudsen JD, Lyytikäinen O, Dryden M, Kennedy KJ, Valiquette L, Pinholt M, Jacobsson G, Laupland KB. The changing epidemiology of group B streptococcus bloodstream infection: a multi-national population-based assessment. Infect Dis (Lond). 2016;48(5):386–91.
Lamagni TL, Keshishian C, Efstratiou A, Guy R, Henderson KL, Broughton K, Sheridan E. Emerging trends in the epidemiology of invasive group B streptococcal disease in England and Wales, 1991–2010. Clin Infect Dis. 2013;57(5):682–8.
Teatero S, McGeer A, Li A, Gomes J, Seah C, Demczuk W, Martin I, Wasserscheid J, Dewar K, Melano RG, et al. Population structure and antimicrobial resistance of invasive serotype IV group B Streptococcus, Toronto, Ontario, Canada. Emerg Infect Dis. 2015;21(4):585–91.
Björnsdóttir ES, Martins ER, Erlendsdóttir H, Haraldsson G, Melo-Cristino J, Kristinsson KG, Ramirez M. Changing epidemiology of group B streptococcal infections among adults in Iceland: 1975–2014. Clin Microbiol Infect. 2016;22(4):e379379–379316.
Francois Watkins LK, McGee L, Schrag SJ, Beall B, Jain JH, Pondo T, Farley MM, Harrison LH, Zansky SM, Baumbach J, et al. Epidemiology of Invasive Group B Streptococcal infections among nonpregnant adults in the United States, 2008–2016. JAMA Intern Med. 2019;179(4):479–88.
Quincer EM, Cranmer LM, Kamidani S. Prenatal maternal immunization for Infant Protection: a review of the vaccines recommended, infant immunity and future research directions. Pathogens 2024, 13(3).
Carreras-Abad C, Ramkhelawon L, Heath PT, Le Doare K. A vaccine against Group B Streptococcus: recent advances. Infect Drug Resist. 2020;13:1263–72.
Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J Microbiol Methods. 2010;80(2):212–4.
Kannika K, Pisuttharachai D, Srisapoome P, Wongtavatchai J, Kondo H, Hirono I, Unajak S, Areechon N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J Appl Microbiol. 2017;122(6):1497–507.
Huebner EM, Gudjónsdóttir MJ, Dacanay MB, Nguyen S, Brokaw A, Sharma K, Elfvin A, Hentz E, Rivera YR, Burd N, et al. Virulence, phenotype and genotype characteristics of invasive group B Streptococcus isolates obtained from Swedish pregnant women and neonates. Ann Clin Microbiol Antimicrob. 2022;21(1):43.
Slotved HC, Møller JK, Khalil MR, Nielsen SY. The serotype distribution of Streptococcus agalactiae (GBS) carriage isolates among pregnant women having risk factors for early-onset GBS disease: a comparative study with GBS causing invasive infections during the same period in Denmark. BMC Infect Dis. 2021;21(1):1129.
Choi SJ, Kang J, Uh Y. Recent epidemiological changes in Group B Streptococcus among pregnant Korean women. Ann Lab Med. 2021;41(4):380–5.
Yoon IA, Jo DS, Cho EY, Choi EH, Lee HJ, Lee H. Clinical significance of serotype V among infants with invasive group B streptococcal infections in South Korea. Int J Infect Dis. 2015;38:136–40.
Kang HM, Lee HJ, Lee H, Jo DS, Lee HS, Kim TS, Shin JH, Yun KW, Lee B, Choi EH. Genotype characterization of Group B Streptococcus isolated from infants with Invasive diseases in South Korea. Pediatr Infect Dis J. 2017;36(10):e242–7.
Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, Madhi SA, Baker CJ, Bartlett L, Cutland C, et al. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: systematic review and Meta-analyses. Clin Infect Dis. 2017;65(suppl2):S160–72.
Ji W, Liu H, Madhi SA, Cunnington M, Zhang Z, Dangor Z, Zhou H, Mu X, Jin Z, Wang A, et al. Clinical and molecular epidemiology of Invasive Group B Streptococcus Disease among infants, China. Emerg Infect Dis. 2019;25(11):2021–30.
Margarit I, Rinaudo CD, Galeotti CL, Maione D, Ghezzo C, Buttazzoni E, Rosini R, Runci Y, Mora M, Buccato S, et al. Preventing bacterial infections with pilus-based vaccines: the group B streptococcus paradigm. J Infect Dis. 2009;199(1):108–15.
Lu B, Wu J, Chen X, Gao C, Yang J, Li Y, Wang J, Zeng J, Fang Y, Wang D, et al. Microbiological and clinical characteristics of Group B Streptococcus isolates causing materno-neonatal infections: high prevalence of CC17/PI-1 and PI-2b sublineage in neonatal infections. J Med Microbiol. 2018;67(11):1551–9.
Tsai IA, Su Y, Wang YH, Chu C. Alterations in genes rib, scpB and Pilus Island decrease the prevalence of predominant serotype V, not III and VI, of Streptococcus agalactiae from 2008 to 2012. Pathogens 2022, 11(10).
Zhang L, Ma L, Zhu L, Zhou XH, Xu LJ, Guo C, Meng JH, Zhang XH, Liu QH, Huang R. Molecular characterization of pathogenic group B streptococcus from a tertiary hospital in Shanxi, China: high incidence of sequence type 10 strains in infants/pregnant women. J Microbiol Immunol Infect. 2021;54(6):1094–100.
Springman AC, Lacher DW, Waymire EA, Wengert SL, Singh P, Zadoks RN, Davies HD, Manning SD. Pilus distribution among lineages of group b streptococcus: an evolutionary and clinical perspective. BMC Microbiol. 2014;14:159.
Lazzarin M, Mu R, Fabbrini M, Ghezzo C, Rinaudo CD, Doran KS, Margarit I. Contribution of pilus type 2b to invasive disease caused by a Streptococcus agalactiae ST-17 strain. BMC Microbiol. 2017;17(1):148.
Liu Y, Liu J. Group B Streptococcus: virulence factors and pathogenic mechanism. Microorganisms 2022, 10(12).
Al Safadi R, Mereghetti L, Salloum M, Lartigue MF, Virlogeux-Payant I, Quentin R, Rosenau A. Two-component system RgfA/C activates the fbsB gene encoding major fibrinogen-binding protein in highly virulent CC17 clone group B Streptococcus. PLoS ONE. 2011;6(2):e14658.
Delara M, Vadlamudi NK, Sadarangani M. Strategies to prevent early and Late-Onset Group B Streptococcal Infection via interventions in pregnancy. Pathogens 2023, 12(2).
Heath PT. Status of vaccine research and development of vaccines for GBS. Vaccine. 2016;34(26):2876–9.
Martins ER, Andreu A, Correia P, Juncosa T, Bosch J, Ramirez M, Melo-Cristino J. Group B Streptococci causing neonatal infections in barcelona are a stable clonal population: 18-year surveillance. J Clin Microbiol. 2011;49(8):2911–8.
Acknowledgments
We are grateful to microbiology laboratory staff for providing the bacterial isolates.
Funding
This study was supported by a research grant from Jeju National University Hospital. (No. 2020-01)
Author information
Authors and Affiliations
Contributions
Study design: JHC and HLAnalysis of samples: JHC, THK and YRKCollection and analysis of data: JHC, THK and ETKOriginal writing: JHCRevision of manuscript: HL.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved and a written consent was waived by the Institutional Review Board of Jeju National University Hospital (approved no. 2020-06-019). This study was conducted in accordance with the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Choi, J.H., Kim, T.H., Kim, E.T. et al. Molecular epidemiology and virulence factors of group B Streptococcus in South Korea according to the invasiveness. BMC Infect Dis 24, 740 (2024). https://doi.org/10.1186/s12879-024-09625-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09625-1