Abstract
Background
We aimed to assess serum 25-hydroxyvitamin D3 (25(OH)D3) concentrations in extrapulmonary tuberculosis (EPTB) patients and to evaluate the effect of vitamin D3 supplementation on their treatment course.
Methods
Serum 25(OH)D3concentrations were measured in 47 newly diagnosed EPTB patients and 42 controls. Vitamin D-deficient EPTB patients were randomly assigned to receive 50,000 IU of vitamin D3 (cholecalciferol) orally once a week for 6 weeks (total 300,000 IU), followed by maintenance doses of 1000 IU a day besides anti-TB drugs or the first line anti-TB treatment only. Follow up serum 25(OH)D3 concentrations were measured after 3 months of starting vitamin D3 supplementation. Both groups were evaluated for clinical, laboratory, and radiological outcomes after treatment.
Results
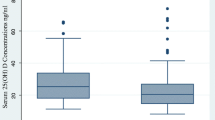
Serum 25(OH)D3 concentrations were significantly lower among TB cases (17.1 ± 5.5 nmol/L) compared to healthy controls (51.8 ± 27.3 nmol/L), and vitamin D deficiency was observed in all EPTB patients (n = 47). Patients in VD3 supplementation group had significantly higher weight gain and serum albumin level at 2 months and end of treatment, higher hemoglobin concentration at the end of treatment, significantly lower CRP and ESR at 2 months and at the end of treatment. In cases with TB pleurisy, a significant higher rate of full resolution of pleural fluid after 6 months of anti-TB treatment and shorter treatment duration were noted compared to the other group.
Conclusions
Vitamin D deficiency is prevalent in EPTB patients, in whom, vitamin D supplementation is a useful adjunctive therapy to anti-TB drugs and improves treatment course.
Similar content being viewed by others
Introduction
Tuberculosis (TB) is a major public health threat and a leading cause of morbidity and mortality worldwide. The World health organization (WHO) estimates that there were 10.6 million incident cases of TB, and 1.6 million deaths due to TB in 2021 [1]. Egypt is ranked among the mid-level incidence countries. According to a WHO estimation of the TB burden in 2021, the incidence rate of TB cases was 10 cases per 100,000 inhabitants [2].
Several factors that could possibly affect the incidence and progression of TB have been identified; one of them is vitamin D deficiency [3].Vitamin D is an immunomodulatory micronutrient that affects both innate and adaptive immune responses [4]. Numerous studies have shown that vitamin D deficiency plays a role in TB prevalence, susceptibility to active disease and worse disease outcome in different populations [5, 6].
Two major forms of vitamin D, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are present naturally in small amounts in a few food sources, mainly oily fish for vitamin D3 and mushrooms and egg yolks for vitamin D2 [7].
D2 and D3 are structurally similar, but D2 has an extra methyl group and double bonds. However, these structural differences do not affect the metabolic activation of vitamin D; therefore, both forms are considered equivalent [8]. Vitamin D2 is most commonly added to foods given the paucity of naturally occurring VD-rich foods, whereas vitamin D3 is mainly synthesized in the skin during exposure to ultra violet B (UV-B) radiation from 7-dehydrocholesterol, but also can be obtained from dietary intake [9]. Subsequently, two hydroxylation reactions occur in the liver and kidney to form 25-hydroxyvitamin D3 and the active hormone form of vitamin D, 1,25-dihydroxyvitamin D3 (calcitriol) by essential enzymes 25- and 1-α-hydroxylases, respectively [9]. 1-α-hydroxylase is predominantly found in the proximal tubular cells of the kidney but has also been described in many other cell types including skin, brain, prostate, pancreas and macrophages [10].
In 2006, Liu and colleagues showed that the triggering of toll-like receptors (TLRs) on the cell surface of human macrophages by tuberculosis protein caused the upregulation of genes leading to the production of the vitamin D receptor (VDR), a polymorphic nuclear receptor that regulates the expression of genes crucial for immune function and involved in cytokine production and the vitamin D-1-α-hydroxylase enzyme, leading to both increased levels of active vitamin D and increased potential binding of calcitriol with the VDR [11]. Calcitriol is then induces the production of several endogenous antimicrobial peptides, particularly cathelicidin LL-37 and defensin beta2 [11]. Additionally, it induces oxidative species, including NO and H2O2 [12] and suppresses matrix metalloproteinase enzymes linked to the pathogenesis of pulmonary cavitation [13]. The resulting increased cathelicidin production, which is totally dependent on vitamin D to be produced, leads to killing of intracellular mycobacterium tuberculosis [14].
In the pre-antibiotic era, cod liver oil, UVB phototherapy, sunshine, oral vitamin D, and injectable vitamin D were all shown to be able to safely treat tuberculosis [15]. The Nobel prize in medicine in 1903 was awarded to Dr. Neils Ryberg Finsen acknowledging his success in curing hundreds of long-standing cases of lupus vulgaris (cutaneous tuberculosis infections) with refracted light rays from an electric arc lamp, and this therapeutic approach became the standard of care for treating TB until the discovery of antibiotics in the 1940’s [15]. Furthermore, several reports from the 1940s document cases where patients with lupus vulgaris were safely and effectively cured by taking daily doses of 100,000 IU to 150,000 IU of oral vitamin D2 as the sole treatment for 2 to 3 months, all without developing complications related to hypercalcemia or withdrawing from therapy [16,17,18,19].
However, randomized clinical trials of vitamin D supplementation in patients with pulmonary TB performed over the past few decades have produced mixed findings on sputum culture/smear conversion and other TB treatment outcomes [20,21,22,23,24,25,26,27,28]. In addition, to the best of our knowledge; no randomized trials have examined the effect of vitamin D supplementation on the treatment of extrapulmonary TB (EPTB). We conducted the present study to assess serum 25-hydroxyvitamin D3 (25(OH)D3) concentrations in newly diagnosed patients with EPTB, its relation to disease activity and worse disease outcome and the effect of vitamin D3 supplementation on response to anti-TB treatment in EPTB patients.
Materials and methods
Study design and participants
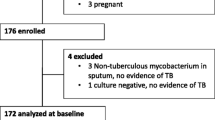
A total of 47 EPTB patients who presented to the TB outpatient clinic, at Cairo University Hospital, Egypt were consecutively recruited over the period from November 2020 till September 2021 and followed up for 6–12 months after starting anti-TB treatment. The study was designed in two phases: the first phase was a case-control study aiming at assessing serum 25(OH)D3 concentrations in newly diagnosed EPTB patients compared with healthy controls, and the second phase was a pilot randomized controlled clinical trial to evaluate the role of combined vitamin D3 supplementation to standard TB therapy in the treatment course of EPTB patients compared to standard therapy alone.
Inclusion criteria were: [1] adult patients (age ≥ 18 years old); [2] Naïve for anti-TB treatment. As per WHO guidelines [29, 30], the diagnosis of EPTB cases was confirmed according to the following: (a) Microbiological evidence in form of acid-fast bacilli (AFB) positivity on tissue or fluid staining, growth of AFB on culture of tissue or fluid or positive GeneXpert MTB/RIF testing on tissue or fluid sample; (b) Histological findings of epithelioid cell granulomas with caseous necrosis in tissue biopsies. Healthy individuals who did not have any history of TB or contacts with TB patients and had no symptoms or signs of TB or pathologic findings in radiology were included in control group. These volunteers were matched to patients by age (± 5years), gender and season (± 1 month). The exclusion criteria were subjects with a history of diabetes mellitus (DM), hepatic disease, renal failure, malignancy, human immunodeficiency virus (HIV) infection, multi-drug resistant TB (MDR-TB), pregnancy and lactation, sarcoidosis, hyperparathyroidism or those taking vitamin D supplementation in the previous 12 months, medications that affect vitamin D levels, corticosteroids, or immunosuppressive agents. This study was approved by the Research Ethics Committee, Faculty of Medicine, Cairo University (Approval code: MD-241-2020).Written informed consent was taken from all the participants.
Study procedure
Phase I
In phase I, all the participants were subjected to detailed history taking including demographic data, comorbidities, concomitant medications, smoking, intravenous (IV) drug use, and alcohol intake. EPTB cases were also assessed for TB associated constitutional symptoms (i.e. fever, night sweats, loss of weight, loss of appetite, fatigue) and local symptoms (e.g. cough, shortness of breath, chest pain, enlarged peripheral lymph node, abdominal pain, diarrhea, bleeding per rectum, abdominal distention …etc ). Height and weight were measured, and body mass index (BMI) was calculated using the formula: weight (kg)/height (m2).
Laboratory evaluation included complete blood count (CBC), liver biochemical profile (total serum bilirubin, liver enzymes [alanine aminotransferase (ALT), aspartate aminotransferase (AST)], and serum albumin), serum creatinine, and serum calcium. For cases, baseline inflammatory markers (Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)) were also measured. Albumin-adjusted serum calcium concentrations were calculated using the following equation: calcium (mg/dL) = total calcium (mg/dL) + 0.8 × [4 - albumin (g/dL)]. Hypocalcemia, normo-calcemia or hypercalcemia were defined when corrected serum calcium levels were below 8.5 mg/dl, 8.5–10.10 mg/dl or exceeding 10.10 mg/dl, respectively [31]. All chemistry analysis was performed on Beckman Coulter AU680 autoanalyzer according to the manufacturer’s methods.
Measurement of serum 25(OH)D3 concentration
We measured serum concentrations of 25(OH)D3 for cases, before starting anti- TB therapy, and for controls using High Performance Liquid Chromatography (HPLC-UV method). It has sensitivity up to: 1.5 ng/mL with a linearity: 2–500 ng/mL for detection. To convert ng/mL to nmol/L, multiply by 2.496. Serum 25(OH)D3 concentrations < 30 nmol/L were defined as vitamin D deficiency, 25(OH)D3 concentrations from 30 to 50 nmol/L were considered as insufficient, and serum 25(OH)D3 concentrations > 50 nmol/L were considered normal [32].
All EPTB patients underwent radiological investigations according to TB site including: (a) abdominal ultrasonography to examine amount of ascites, septations or loculation, peritoneal thickening, thickened omentum, mesenteric thickening, intestinal involvement, any focal lesion or enlarged abdominal lymph nodes; (b) Lymph node (LN) sonography to assess location, size, number, shape, and echogenicity of enlarged LNs, formation of fistulae and/or abscesses, as well as any change in the size of nodes during anti-TB treatment; (c) Computed tomography (CT) in TB pleurisy cases to assess the amount of pleural effusion, pleural thickening, and LN enlargement.
Phase II
In phase II, EPTB patients with concomitant Vitamin D deficiency were randomly assigned into two groups: Group I (“Vitamin D” group) included patients who received both vitamin D supplementation and the first line anti-tuberculous treatment. Vitamin D supplementation was given according to the recommended dose [33] i.e. 50,000 IU vitamin D3 (cholecalciferol) orally once a week for 6 weeks (total 300,000 IU), followed by maintenance doses of 1000 IU a day. The first line anti-TB treatment included [ Isoniazide (5 mg/kg /day PO), Rifampin (10 mg/kg/day PO), Ethambutol (15 mg/kg/day PO) and Pyrazinamide(15–30 mg/kg/day PO)] for 2 months then Isonizide and Rifampin for 4–10 months; while Group II (“No vitamin D”) received the first line anti-TB treatment only. Follow up serum 25(OH)D3 concentrations were measured after 3 months of starting vitamin D3 supplementation. Participants were randomized using identical sealed envelopes technique.
Outcome measures
Response to anti-TB treatment was compared between the two studied groups at two time points; after the intensive phase of anti-TB treatment at 2 months and at the end of treatment, according to the following parameters;
-
i.
Improvement of (constitutional and local) symptoms. The number of symptoms the participants reported at baseline and symptom count ratio (SCR) derived by dividing the number of symptoms reported as much better or resolved at the follow-up visit by the total number of symptoms reported at baseline.
-
ii.
Changes in weight: patients were weighed at each follow-up visit, and weight change was recorded in kilograms (kg) and as percentage change in weight at 2 months and end of treatment compared to baseline.
-
iii.
Follow up laboratory investigations including; CBC, liver biochemical profile (AST, ALT, and serum albumin), inflammatory markers (ESR and CRP).
-
iv.
Change in serum 25(OH)D3 concentrations (in the patient group who received vitamin D3 supplementation besides the first-line anti-TB drugs) three months after starting vitamin D3 supplementation.
-
v.
Follow up radiological investigations repeated at 2 months, 6months and at the end of treatment to evaluate regression of lymph nodes size by LN ultrasound, amount pleural effusion by CT chest and regression of amount peritoneal effusion by abdominal ultrasound.
Adverse effects
Patients who received vitamin D3 supplement were questioned for the following adverse effects related to hypercalcemia e.g. anorexia, nausea, vomiting, excessive thirst, symptoms of kidney stones, and confusion. Serum levels of calcium were measured at the end of 2nd month.
Statistical methods
Data were statistically described in terms of mean ± standard deviation (± SD), median and range, or frequencies and percentages when appropriate. Numerical data were tested for the normal assumption using Kolmogorov Smirnov test. Comparison of numerical variables between the study groups was done using Student t test for independent samples in comparing normally distributed data and Mann Whitney U test for independent samples for comparing non-normal data. Over time comparison of numerical variables was done using paired t test for normally distributed data and Wilcoxon signed rank test for paired (matched) samples for non-normal data. For comparing categorical data, Chi-square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. Over time categorical data were compared using McNemar test. Two-sided p values less than 0.05 was considered statistically significant. IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, USA) release 22 for Microsoft Windows was used for all statistical analyses.
Results
This study included 47 EPTB patients and 42 healthy controls. Table 1 shows the baseline characteristics and laboratory data of study participants. Mean age for TB cases was 32.0 ± 10.6 years, 53.2% were males. Compared to controls, EPTB patients were more often unemployed (P = 0.011) and had significantly lower BMI (p < 0.001), significantly lower hemoglobin concentration and lower serum albumin levels (P = 0.023 and P = < 0.001, respectively), significantly lower serum 25(OH)D3 concentrations (17.1 ± 5.5 nmol/L) compared to healthy controls (51.8 ± 27.3 nmol/L, p < 0.001). Vitamin D deficiency was observed in all EPTB cases (n = 47, 100.0%), while in the control group, only 9.5% had vitamin D deficiency, and 59.5% had vitamin D insufficiency. Table 2 shows the distribution of extrapulmonary TB among TB cases (n = 47); 38.3% had TB pleurisy, 34% had TB lymphadenitis, 17% had TB peritonitis and 5 patients (10.6%) had both TB peritonitis and pleurisy.
In phase II of the study, EPTB patients were randomly assigned to either the “Vitamin D” group or the “No vitamin D” group. Both groups had similar baseline characteristics. Out of the 47 TB cases, 1 patient (2.1%) died and 4 (8.5%) were lost-to-follow-up before first follow-up visit. Therefore, a total of 42 EPTB patients were followed during treatment, 21 patients in each group (Table 2).
The main reported symptoms at baseline (n = 42) were fatigue (100%), fever (95.2%), weight loss (92.9%), night sweats (73.8%), loss of appetite (69%), and local symptoms related to the site of TB infection such as peripheral lymph node swelling (14/42,33.3%), abdominal pain (12/42, 28.6), abdominal distension (12/42, 28.6%),chest pain (5/42,11.9%), dyspnea(17/42, 40.5%),and cough(6/42, 14.3%) (Table 3).
All 42 patients showed improvement in presenting symptoms after two months of treatment and complete resolution at the end of the anti-TB therapy, with no significant difference between the two groups regarding the number of symptoms reported at baseline, number of improved symptoms at 2 months and at the end of treatment (p = 0.282, 0.254 and 0.282) as well as symptom count ratio (SCR) at 2 months and at the end of treatment. Moreover, there was no significant difference in the mean treatment duration between both groups (p = 0.065) except in subgroup of patients with TB pleurisy, in which the patients in VD3 supplementation group had shorter treatment duration (p = 0.021) (Table 4).
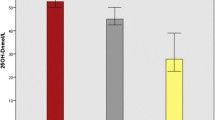
An overall significant weight gain was observed in all TB cases at 2 months and at treatment completion compared to baseline, with patients in VD3 supplementation group had significantly higher mean weight gain (4.4 ± 1.8 kg vs. 3.1 ± 1 kg at 2 months (p = 0.004) and 9.5 ± 3 kg vs. 7.4 ± 1.7 kg at the end of TB treatment (p = 0.010)) and percentage of weight gain (7.4 ± 2.9%vs. 5 ± 1.6% at 2 months(p = 0.002) and 16.3 ± 6%vs. 12.5 ± 4% at the end of treatment(p = 0.023) (Fig. 1a& b).
As shown in Table 5, patients in VD3 supplementation group had significantly higher hemoglobin concentration at the end of treatment (p < 0.001),higher serum albumin level at 2 months and at the end of treatment(p = 0.007 and P < 0.001), lower CRP level (p = 0.002 and P < 0.001) and lower ESR at 2 months and at the end of treatment(p = 0.02 and P < 0.001). Additionally, patients in VD3 supplementation group had a significant increase in serum 25(OH)D3 concentrations after 3 months of supplementation (71.6 ± 7.1nmol/L)compared to baseline (17.3 ± 5.6nmol/L, p < 0.001). None of the patients experienced hypercalcemia or any adverse events related to anti-tuberculosis treatment.
As shown in Table 6, all cases with TB lymphadenitis demonstrated a significant regression in lymph node size at the end of anti-TB treatment compared to the baseline (p < 0.001), with no significant differences between the “Vitamin D” and the “No vitamin D” groups. Likewise, regression in amount of ascites was detected in 75% of cases with TB peritonitis (n = 9/12) at 2 months and in all cases after 6 months of anti-TB treatment, however, with no complete resolution of ascites. So, treatment was extended for additional 3 months in 10 patients (83.3%) and for additional 6 months in 2 patients (16.7%), with no significant differences between the “Vitamin D” and the “No vitamin D” groups. In cases with TB pleurisy, regression of pleural effusion was noted in all patients at 2 months of anti-TB therapy, and full resolution of effusion occurred in 14.3% and 81% of patients at 2 months and 6 months, respectively, with higher rate of resolution of pleural fluid at 6 months in the “Vitamin D” group (100% vs. 60%, p = 0.020).
Discussion
This study revealed a high prevalence of vitamin D deficiency among newly diagnosed patients with EPTB. TB cases showed significantly lower vitamin D levels compared to their age and sex-matched healthy controls. Our findings are consistent with earlier studies demonstrated that active pulmonary TB patients had lower serum vitamin D levels compared to their household contacts and healthy controls [5, 20, 21]. Also, Hammami et al. [34] reported that vitamin D levels were significantly lower among cases of EPTB in comparison with controls and vitamin D deficiency was an independent predictor of EPTB. Similarly, Balgi. et al. [35] found lower vitamin D concentrations in both extrapulmonary and pulmonary TB cases with patients with TB meningitis had significantly lower levels of vitamin D in comparison with other forms of TB. Another study by Pareek et al. [36] showed that the patients with EPTB had lower mean vitamin D (25-OH D) concentration as compared with pulmonary TB and doubling in serum vitamin D concentration significantly reduced the risk of EPTB.
This association between vitamin D deficiency and pulmonary or extrapulmonary TB could be explained by the immunomodulatory role for vitamin D. It plays a crucial role in regulating both adaptive and innate host immune defense against TB. Vitamin D enhances the phagocytic capacity and granuloma formation of monocytes and macrophages, and increases the production of antimicrobial peptides such as cathelicidin, leading to killing of intracellular mycobacterium tuberculosis [15].
Low serum 25(OH)D3 concentrations in EPTB patients in our study may be attributed to several mechanisms such as inadequate dietary intake as reflected by the high frequency of low BMI and higher rates of unemployment among the TB patients indicating lower socioeconomic status. Moreover, 80.9% of TB patients were living in urban areas. Increasing urbanization has been associated with tendency to spend most time indoors, leading to insufficient exposure to sunlight. Additionally, the TB disease itself might have led to restricted physical activity, lack of adequate exposure to sunlight and consequent low concentrations of vitamin D. Another hypotheses that could explain vitamin D deficiency in TB cases is that vitamin D behaves as a negative acute phase reactant in TB, as has been documented in other conditions such as surgery [37] and during immune restoration syndrome in TB-HIV co-infection [38].In addition, Vitamin D level is estimated by measuring serum 25(OH)D3 concentrations. The 25(OH)D3level mainly reflects protein-bound vitamin D and may not represent the level of free active form of vitamin D. Consequently, during inflammation, similarly to levels of several other blood acute-phase proteins, the concentration of albumin decreases and might modify 25(OH)D3level results [39].
Baseline laboratory parameters in our study showed significantly lower albumin levels and hemoglobin concentrations in TB patients as compared with healthy controls. Low serum albumin levels is attributed to severe malnutrition associated with TB as well as albumin is a negative acute phase protein, so its concentration decreases in the context of significant inflammation and infection as in TB [40]. Several previous studies have demonstrated that anemia is highly prevalent in active TB cases and can be attributed to iron deficiency secondary to TB associated malnutrition and iron depletion [41].
In phase II of the study, we investigated the effect of vitamin D supplementation when added to the first line anti-TB drugs on response to treatment parameters including clinical improvement, laboratory, and radiological parameters in patients with EPTB.
An overall clinical improvement was noted in all EPTB patients, with significant weight gain during and after anti-TB treatment. The administration of vitamin D3 supplementation did not affect the overall treatment duration, except in the subgroup of patients with TB pleurisy who had shorter treatment duration.
Patients in vitamin D3 supplementation group had a greater weight gain during and at the end of TB treatment, compared to the group who received only anti-TB drugs.
Several studies have indicated that weight gain is associated with successful treatment outcomes in patients with pulmonary and extrapulmonary TB [42,43,44]. Conversely, lesser weight gain or weight loss were associated with poor outcomes [42, 43], or relapse [45]. In a recent study [44] evaluating treatment response in extrapulmonary tuberculosis in a low-resource setting, in TB cases with successful treatment outcomes, ≥ 5% weight gain was noted in 73 and 83% of the cases at 2 months and at treatment completion.
Furthermore, patients in vitamin D3 supplementation group showed significant improvements in hemoglobin concentration, serum albumin levels, as well as lower inflammatory markers; ESR and CRP level, both at 2 months and at the end of TB treatment. Radiological assessment demonstrated positive outcomes with vitamin D3 supplementation, particularly in patients with TB pleurisy who had a higher rate of full resolution of pleural fluid after 6 months of anti-TB treatment. Also, among TB peritonitis cases in vitamin D3 supplementation group, a higher percentage of patients experienced mild ascites at both 2 months and 6 months, though this was not statistically significant.
Compared to our results, studies investigated the effect of vitamin D supplementation on the treatment course of active pulmonary TB demonstrated variable results.
In a study by Nursyam et al. [22], pulmonary TB patients given a daily dose of vitamin D at 10,000 IU (250 mcg) for 6 weeks had significantly higher sputum conversion rates and radiological improvement compared to the placebo group. Similarly, Kota et al. [23] reported that in type 2 diabetes patients with pulmonary TB receiving 60,000 IU of vitamin D3 supplementation per week alongside anti-TB treatment, the duration of sputum conversion to 100% negative for AFB was 6 weeks compared to 8 weeks in the control group.
Salahuddin et al. [24] reported greater weight gain and accelerated radiological recovery in pulmonary TB patients who received two doses of 600,000 IU vitamin D3 administered intramuscularly, in comparison to the placebo group. Also, Hassanein., et al. [20] demonstrated that administration of single dose of 200,000IU vitamin D3 during pulmonary TB treatment has resulted in a rapid decline in sputum conversion time compared to the group receiving standard anti-TB treatment alone. Sato and colleagues [21] reported that in cured patients with active pulmonary TB, serum vitamin D levels showed a significantly negative correlation with time duration until sputum conversion, as well as platelets count and CRP and a significant positive correlation with serum albumin. They stated that these correlations reflect the anti-inflammatory effect of vitamin D and suggest that a low serum vitamin D level may not only be a risk factor for the development of active TB but it may also be related to poor treatment outcomes in active TB patients [21].
However, Martineau et al. [25] reported that the administration of vitamin D3 supplementation in four fortnightly doses of 2.5 mg (100,000 IU) had no effect on time to sputum culture conversion. However, vitamin D supplementation accelerated normalization of ESR and serum CRP and reduced chemokine production [26]. Additionally, two randomized-controlled clinical trials conducted by Wejse et al. [27] and Ganmaa et al. [28] showed that after administration of three doses of 100,000IU vitamin D2 given at 0, 5 and 8 months and four biweekly doses of 3.5 mg (140,000 IU) vitamin D3 respectively, no significant difference in the rate of mortality and sputum clearance was recognized in comparison with placebo group. Also, Ganmaa et al. [28] reported that no significant effect of vitamin D supplementation was seen on serum concentrations of the acute-phase reactants CRP, albumin, or ESR.
Our study reported normal serum calcium levels and no significant difference before and after vitamin D3 supplementation. This finding aligns with earlier studies that explored higher doses of vitamin D supplementation in the treatment course of TB with no reported hypercalcemia [22, 46, 47]. Studies have shown that there is a wide margin of safety, as serum 25(OH)-vitamin D concentrations > 400 ng/ml appear to be necessary before hypercalcemia develops [48, 49]. Compared to our results, Hassanein et al. [20] found a statistical significant difference between serum calcium at the start of anti-TB treatment and that measured at the end of 2nd month after initiation of vitamin D therapy, with elevated serum calcium level detected in 36.7% of patients. However, this was asymptomatic in all cases.
To the best of our knowledge, this is the first study to evaluate the role of vitamin D3 supplementation in the treatment course of patients with EPTB (including patients with TB lymphadenitis, TB pleurisy and TB peritonitis). However, the study has some limitations, being a single centre study and the small sample size limit the generalizability of the results. Therefore, studies with larger sample sizes that recruit patients with other forms of EPTB are still needed.
Conclusions
This study showed high prevalence of vitamin D deficiency among newly diagnosed patients with EPTB, compared to healthy controls. Additionally, vitamin D3 supplementation has been beneficial in treatment of EPTB, and associated with higher weight gain, as well as better improvement of serum concentrations of hemoglobin and acute-phase reactants. Therefore, we recommend assessing vitamin D level in TB patients starting anti-TB therapy, followed by administration of vitamin D supplementation according to the level of vitamin D insufficiency/deficiency detected.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- EPTB:
-
extrapulmonary tuberculosis
- TB:
-
Tuberculosis
- WHO:
-
World health organization
- AFB:
-
acid-fast bacilli
- MDR-TB:
-
multi-drug resistant TB
- BMI:
-
body mass index
- CRP:
-
C reactive protein
References
World Health Organization. Global tuberculosis report 2022. Geneva. Switzerland: World Health Organization; 2022. https://www.who.int/teams/global-tuberculosis-programme/data.
World Health Organization. WHO, Egypt tuberculosis profile 2021. Avilable from: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22EG%22
Junaid K, Rehman A. Impact of vitamin D on infectious disease-tuberculosis-a review. Clin Nutr Experimental. 2019;25:1–0.
Sanlier N, Guney-Coskun M, Vitamin D. The immune system, and its relationship with diseases. Egypt Pediatr Association Gaz. 2022;70(1):39.
Sutaria N, Liu CT, Chen TC. Vitamin D status, receptor gene polymorphisms, and supplementation on tuberculosis: a systematic review of case-control studies and randomized controlled trials. J Clin Translational Endocrinol. 2014;1(4):151–60.
Zeng J, Wu G, Yang W, Gu X, Liang W, Yao Y, Song Y. A serum vitamin D level < 25nmol/l pose high tuberculosis risk: a meta-analysis. PLoS ONE. 2015;10(5):e0126014.
Reijven PL, Soeters PB. Vitamin D: a magic bullet or a myth? Clin Nutr. 2020;39(9):2663–74.
Khammissa RA, Fourie J, Motswaledi MH, Ballyram R, Lemmer J, Feller L. The biological activities of vitamin D and its receptor in relation to calcium and bone homeostasis, cancer, immune and cardiovascular systems, skin biology, and oral health. BioMed research international. 2018;2018.
Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–29.
Dusso AS, Brown AJ, Slatopolsky E, Vitamin D. Am J Physiology-Renal Physiol. 2005;289.
Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3.
Sly LM, Lopez M, Nauseef WM, Reiner NE. 1α, 25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276(38):35482–93.
Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, Newton SM, Wilkinson KA, Davidson RN, Griffiths CJ, Wilkinson RJ. 1α, 25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127(4):539–48.
Gombart AF. The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–65.
McCullough PJ, Lehrer DS, Vitamin D. Cod liver oil, sunshine, and phototherapy: safe, effective and forgotten tools for treating and curing tuberculosis infections—A comprehensive review. J Steroid Biochem Mol Biol. 2018;177:21–9.
Dowling GB, Prosser Thomas EW. Lupus vulgaris treated with calciferol. Proc R Soc Med. 1945;39:96–9.
Dowling GB, Prosser Thomas EW, Wallace HJ. Lupus Vulgaris treated with Calciferol. Proc R Soc Med. 1946;39(5):225–7.
Michelson HE, Steves RJ. Treatment of cutaneous tuberculosis with large doses of vitamin D2. Archives Dermatology Syphilology. 1947;56(3):317–24.
Tomlinson KM. Calcium content of skin in lupus vulgaris treated with calciferol. Lancet. 1948;254:327–8.
Hassanein EG, Mohamed EE, Baess AI, El-Sayed ET, Yossef AM. The role of supplementary vitamin D in treatment course of pulmonary tuberculosis. Egypt J Chest Dis Tuberculosis. 2016;65(3):629–35.
Sato S, Tanino Y, Saito J, Nikaido T, Inokoshi Y, Fukuhara A, Fukuhara N, Wang X, Ishida T, Munakata M. The relationship between 25-hydroxyvitamin D levels and treatment course of pulmonary tuberculosis. Respiratory Invest. 2012;50(2):40–5.
Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Hemoglobin. 2006;1500:1500.
Kota SK, Jammula S, Kota SK, Tripathy PR, Panda S, Modi KD. Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diabetes Metabolic Syndrome: Clin Res Reviews. 2011;5(2):85–9.
Salahuddin N, Ali F, Hasan Z, Rao N, Aqeel M, Mahmood F. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT study [Supplementary cholecalciferol in recovery from Tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect Dis. 2013;13(1):1–11.
Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN, Milburn HJ. High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–50.
Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, Timms PM, Venton TR, Bothamley GH, Packe GE, Darmalingam M. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proceedings of the National Academy of Sciences. 2012;109(38):15449-54.
Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–50.
Ganmaa D, Munkhzul B, Fawzi W, Spiegelman D, Willett WC, Bayasgalan P, Baasansuren E, Buyankhishig B, Oyun-Erdene S, Jolliffe DA, Xenakis T. High-dose vitamin D3 during tuberculosis treatment in Mongolia. A randomized controlled trial. Am J Respir Crit Care Med. 2017;196(5):628–37.
World Health Organization. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settings. World Health Organization; 2007.
WHO. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis—rapid diagnostics for tuberculosis detection, 2021 update.
Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. 1973;4(5893):643–6.
Giustina A, Adler RA, Binkley N, Bouillon R, Ebeling PR, Lazaretti-Castro M, Marcocci C, Rizzoli R, Sempos CT, Bilezikian JP. Controversies in vitamin D: summary statement from an international conference. The Journal of Clinical Endocrinology & Metabolism. 2019;104(2):234 – 40.
Francis RM, Aspray TJ, Bowring CE, Fraser WD, Gittoes NJ, Javaid MK, Macdonald HM, Patel S, Selby PL, Tanna N. National Osteoporosis Society practical clinical guideline on vitamin D and bone health. Maturitas. 2015;80(2):119–21.
Hammami F, Koubaa M, Mejdoub Y, Turki M, Ayed HB, Chakroun A, Rekik K, Smaoui F, Jemaa MB. The association between vitamin D deficiency and extrapulmonary tuberculosis: case-control study. Tuberculosis. 2021;126:102034.
Balgi V, Sanjana JM, Suneetha DK, Surendran A, Chandrashekar GS. The study of correlation between vitamin D and tuberculosis in newly detected tuberculosis-pulmonary and extra pulmonary patients attending to KR hospital, Mysuru, Karnataka, India. Int J Adv Med. 2020;7(1):34.
Pareek M, Innes J, Sridhar S, Grass L, Connell D, Woltmann G, Wiselka M, Martineau AR, Kon OM, Dedicoat M, Lalvani A. Vitamin D deficiency and TB disease phenotype. Thorax. 2015;70(12):1171–80.
Waldron JL, Ashby HL, Cornes MP, Bechervaise J, Razavi C, Thomas OL, Chugh S, Deshpande S, Ford C, Gama R. Vitamin D: a negative acute phase reactant. J Clin Pathol. 2013;66(7):620–2.
Conesa-Botella A, Meintjes G, Coussens AK, van der Plas H, Goliath R, Schutz C, Moreno-Reyes R, Mehta M, Martineau AR, Wilkinson RJ, Colebunders R. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012;55(7):1004–11.
Antonelli MJ, Kushner I, Epstein M. The constellation of vitamin D, the acute-phase response, and inflammation. Cleve Clin J Med. 2023;90(2):85–9.
Matos ED, Moreira Lemos AC. Association between serum albumin levels and in-hospital deaths due to tuberculosis. Int J Tuberc Lung Dis. 2006;10(12):1360–6.
Gil-Santana L, Cruz LA, Arriaga MB, Miranda PF, Fukutani KF, Silveira-Mattos PS, Silva EC, Oliveira MG, Mesquita ED, Rauwerdink A, Cobelens F. Tuberculosis-associated anemia is linked to a distinct inflammatory profile that persists after initiation of antitubercular therapy. Sci Rep. 2019;9(1):1381.
Bernabe-Ortiz A, Carcamo CP, Sanchez JF, Rios J. Weight variation over time and its association with tuberculosis treatment outcome: a longitudinal analysis. PLoS ONE. 2011;6(4):e18474.
Hoa NB, Lauritsen JM, Rieder HL. Changes in body weight and tuberculosis treatment outcome in Viet Nam. Int J Tuberc Lung Dis. 2013;17(1):61–6.
Jørstad MD, Dyrhol-Riise AM, Aßmus J, Marijani M, Sviland L, Mustafa T. Evaluation of treatment response in extrapulmonary tuberculosis in a low-resource setting. BMC Infect Dis. 2019;19(1):1–9.
Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR, Tuberculosis Trials Consortium. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med. 2006;174(3):344–8.
Daley P, Jagannathan V, John KR, Sarojini J, Latha A, Vieth R, Suzana S, Jeyaseelan L, Christopher DJ, Smieja M, Mathai D. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15(5):528–34.
Mily A, Rekha RS, Kamal SM, Arifuzzaman AS, Rahim Z, Khan L, Haq MA, Zaman K, Bergman P, Brighenti S, Gudmundsson GH. Significant effects of oral phenylbutyrate and vitamin D3 adjunctive therapy in pulmonary tuberculosis: a randomized controlled trial. PLoS ONE. 2015;10(9):e0138340.
Araki T, Holick MF, Alfonso BD, Charlap E, Romero CM, Rizk D, Newman LG. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J Clin Endocrinol Metabolism. 2011;96(12):3603–8.
McCullough PJ, Lehrer DS, Amend J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: insights from a seven year experience. J Steroid Biochem Mol Biol. 2019;189:228–39.
Acknowledgements
No specific person or organization to be acknowledged.
Funding
Not applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Rasha Eletreby: Sharing in the idea of the study, following all steps of the study, revision of the manuscript. Aisha Elsharkawy: following all steps of the study, revision of the manuscript. Rahma Mohamed: Sharing in the idea of the study, carrying out the clinical part of the study, collecting data, writing the first draft of the paper and is the corresponding author. Mai Hamed: The clinical pathology specialist who did the laboratory work conserning vitamin D level measurement at different stages of the study. Eman Kamal: The pulmonology specialist who followed up the tuberculosis patients clinically. Rabab Fouad: The principal investigator in the study who shared the idea and followed up the whole process of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee, Faculty of Medicine, Cairo University (Approval code: MD-241-2020). Written informed consent was taken from all the participants. The study was carried out in accordance with good clinical practice (GCP) and Helsinki Declaration at 1975 (World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects 2013).
Consent for publication
Not applicable.
Competing interests
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eletreby, R., Elsharkawy, A., Mohamed, R. et al. Prevalence of vitamin D deficiency and the effect of vitamin D3 supplementation on response to anti-tuberculosis therapy in patients with extrapulmonary tuberculosis. BMC Infect Dis 24, 681 (2024). https://doi.org/10.1186/s12879-024-09367-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09367-0