Abstract
Background
Vancomycin-associated acute kidney injury (VA-AKI) is the most clinically relevant side effect of vancomycin. The objective of this study was to investigate the association between VTC and VA-AKI as well as 30-day mortality in critically ill elderly adults.
Method
Elderly patients with trough serum vancomycin concentration records(VTC) in the Medical Information Mart-IV (MIMIC-IV) and eICU databases were retrospectively studied.
Results
A total of 3,146 critically ill elderly adults were finally enrolled. The incidence of VA-AKI in the elderly population was 76.5%. Logistic regression analysis revealed significant relationships between VA-AKI and various factors, including VTC, comorbidities, and laboratory indicators, and SOFA, and GCS score. For each mg/L increase, the OR for VA-AKI increased by 2.5%. The association between VTC and 30-day mortality was found to be statistically significant (odds ratio (OR): 1.021, 95% CI: 1.010–1.031), P < 0.001). The Restricted cubic splines (RCS) curves revealed that VTC ranged of 19.67 to 35.72 mg/l for AKI and 19.17 to 42.86 mg/l for 30-day mortality exhibit OR with 95% CI above 1, indicating statistically significant associations with an increased risk of AKI and 30-day mortality, respectively. In the subgroup analysis, VTC was identified as a risk factor for VA-AKI in specific patient groups, including white individuals, female patients, those with shock, patients with SOFA > 6, patients with baseline creatinine > 1.2 mg/dl and patients with or without exposed to other nephrotoxic medications.
Conclusion
This study found the significant association between VTC and the incidence of VA-AKI and 30-day mortality in critically ill elderly adults. The RCS curves indicated concentration ranges for AKI (19.67–35.72 mg/L) and 30-day mortality (19.17–42.86 mg/L), signifying increased risk.
Similar content being viewed by others
Introduction
Vancomycin, a glycopeptide antibiotic with a substantial bactericidal effect on gram-positive bacterial infections, is commonly used for empiric coverage of methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus sp., Enterococcus sp., Clostridium sp., and Eubacterium sp [1]. It has also been recommended as the first-line treatment for MRSA infections by American, European, and Chinese guidelines [2,3,4,5]. In the USA, almost 10% of hospitalized patients (3.3 million patients) receive vancomycin treatment each year [6, 7].
Vancomycin-associated acute kidney injury (VA-AKI) is the most clinically relevant side effect of vancomycin. Previous studies found that 5%–43% of non-elderly patients exposed to vancomycin developed VA-AKI [8,9,10], which will significantly prolong hospital stays, increase mortality, morbidity, and cost, and may lead to chronic kidney disease [11].
Since the life expectancy of the general population has continued to increase, population aging is a nonnegligible global problem. Renal aging is one of the most noteworthy issues caused by aging. Renal aging can lead to renal function changes, such as a decline in glomerular filtration rate and sodium reabsorption, and renal structural changes, such as a decline in total nephron size and a decrease in nephron number [12]. Therefore, age-related changes in kidney function and structure and multiple comorbidities can increase the susceptibility of elderly adults to VA-AKI [10]. The pathophysiology of VA-AKI remains uncertain. VA-AKI has a dose‒response relationship, and the incidence of VA-AKI increases with higher vancomycin concentrations and doses [13]. Vancomycin is mainly excreted through the kidney. Researchers have found that elderly patients are at risk of VA-AKI [10]. Moreover, elderly people are often complicated with immune system dysfunction and are prone to gram-positive bacterial infections [14, 15]. However, there has been limited published literature on the association of VTC with VA-AKI and 30-day mortality in critically ill elderly patients.
Due to vancomycin nephrotoxicity, vancomycin therapy should be guided by therapeutic drug monitoring (TDM) to reduce VA-AKI risk and optimize effectiveness, particularly in elderly patients. Recent clinical guidelines recommend that vancomycin trough levels should be kept above 10 mg/l and target trough concentration levels should remain at 15–20 mg/l for serious infections caused by MRSA [2,3,4,5]. The objective of this study was to investigate the association between VTC and VA-AKI as well as 30-day mortality in critically ill elderly adults.
Method.
Database
This study was performed using data from the Medical Information Mart for Intensive Care IV (MIMIC-IV) and the eICU collaborative research database [16, 17]. MIMIC-IV, a large, open, and public database, contains numerous anonymized patients who were admitted to the critical care units of Beth Israel Deaconess Medical Center (BIDMC) from 2008 to 2019. The eICU Database is a multicenter database comprising deidentified health data associated with over 200,000 admissions to ICUs across the United States between 2014 and 2015.
MIMIC and eICU databases include vital sign measurements, care plan documentation, comorbid diseases, laboratory tests, microbe findings, severity of illness measures, diagnosis information, and treatment information. The authors have completed ethics courses and gained access to the databases described above.
Study population
The inclusion criteria included the following: 1) elderly patients (age ≥ 65 years), 2). with records of vancomycin treatment, 3). at least one vancomycin trough concentration(VTC) record. The exclusion criteria were as follows: 1) length of stay < 3 days, 2) duration of vancomycin < 2 days, 3) receiving dialysis or extracorporeal membrane oxygenation (ECMO) before vancomycin was administered, and 4) patients with related missing information included VTC record, serum creatinine, vancomycin record. Some patients with missing important information, including certain demographic details (e.g., age, gender), past medical history, blood creatinine levels and urine output for assessing kidney function, were excluded.
Data extraction
The Structured Query Language (SQL) with the PostgreSQL tool (version 9.6) was used to extract data from the MIMIC-IV and eICU databases. The extracted information included age, sex, comorbidities, and laboratory tests. Comorbidities included diabetes, myocardial infarction, chronic pulmonary disease, renal disease, and cerebrovascular disease. We determined the VTC based on the maximum trough concentration observed after obtaining bacterial cultures and after vancomycin administration. During this period, laboratory tests were averaged for each of the patient's indicators. Based on the information above, we calculated sequential organ failure assessment (SOFA) and Glasgow Coma Scale (GCS) scores.
Definition
The primary and secondary outcomes of this study were VA-AKI and 30-day mortality after vancomycin was administered, respectively. We used the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) definition of AKI [18]: An increase in serum creatinine (SCr) by ≥ 0.3 mg/dl within 48 h or an increase in SCr to at least 1.5 times baseline within 1 week or continuous urine output < 0.5 ml/(kg*h). AKI patients were at least stage I in severity. Stage II was defined as a 2–2.9-fold increase in SCr from baseline or continuous urine output < 0.5 ml/(kg*h) for at least 12 h. Stage III was defined as a threefold increase in SCr from baseline, an increase in SCr to ≥ 4 mg/dl, receiving CRRT therapy, continuous urine output < 0.3 ml/(kg*h) for at least 24 h or anuria for at least 12 h. Other nephrotoxic medications included aminoglycosides, first-generation and second-generation cephalosporins, penicillin, tetracycline, rifampicin, Nonsteroidal Anti-Inflammatory Drugs(NSAIDs), amphotericin B, fluconazole, contrast agents, chemotherapeutic drugs like cisplatin and carboplatin, as well as immunosuppressive drugs such as cyclosporine and tacrolimus.
Statistical analysis
Descriptive statistics were presented as either numbers and percentages for categorical variables or means and standard deviations for continuous variables. Continuous variables were compared using Student's t-test for pairwise comparisons and one-way ANOVA for comparisons across multiple groups, while categorical variables were compared pairwise with chi-square test. Regarding vancomycin therapeutic guidelines [5], we divided patients into 4 groups according to trough levels: ≤ 10.0 µg/ml, 10.0–15.0 µg/ml, 15.0–20.0 µg/ml, and > 20 µg/ml. Univariate and multivariate analyses for assessing independent risk predictors were performed using the logistic regression model. In the stepwise logistic regression model using the backwards selection approach, predictor variables were included if their p-values were less than 0.05 and removed if their p-values were greater than 0.10, based on the significance level for variable selection. Restricted cubic splines (RCSs) were employed to investigate the dose–response relationship between the VTC and outcomes, including VA-AKI and 30-day mortality. The RCS analysis was performed using the 25th, 50th, and 75th percentiles of the distribution of troughs as fixed knots. The variables utilized in the RCS analysis were selected based on their statistical significance in the prior multiple logistic regression analysis. Log-rank tests and Kaplan‒Meier curves were also performed. A two-sided P < 0.05 was considered statistically significant. R (version 4.2.1) software and SPSS software (v23.0; IBM, Armonk, NY) were used for statistical analyses.
Results
Characteristics of patients with different trough concentrations and outcomes
As shown in Fig. 1, a total of 3,145 critically ill elderly patients were enrolled for our final data analysis after screening by the inclusion and exclusion criteria. Table 1 showed the characteristics of patients among different VTC groups. White people accounted for most patients enrolled in this study. The mean ages were 77, 76, 75, and 75 for troughs of 5–10, 10.1–15, 15.1–20, and > 20(mg/l), respectively. Compared to other groups, more patients with troughs > 20 mg/l had comorbidities, including congestive heart failure, renal disease, and liver disease. Hemoglobin, serum bilirubin, and creatinine in the > 20 µg/ml group were higher than those in the other groups (p < 0.001). The proportions of patients exposed to other nephrotoxic medications were 13.31% in the 5–10 mg/l group, 13.49% in the 10–15 mg/l group, 22.02% in the 15–20 mg/l group, and 30.00% in the > 20 mg/l group(p < 0.001).The length of stay was also longer in the high vancomycin concentration group. The incidences of VA-AKI and 30-day mortality increased with vancomycin trough concentration levels. The VA-AKI rates were 64.0%, 72.5%, 73.7%, and 82.6% for troughs of 5–10 (mg/l), 10.1–15, 15.1–20, and > 20, respectively. The 30-day mortality rates were 14.4%, 15.9%, 17.4%, and 25.0% for troughs of 5–10, 10.1–15, 15.1–20, and > 20 (mg/l), respectively. The main distribution of pathogen species in patients is described in Supplemental Table S1.
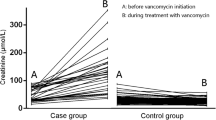
Compared to the non-AKI group, more patients with VA-AKI had comorbidities, including myocardial infarction, congestive heart failure, renal disease, liver disease, and malignant cancer. As shown in Table 2, the vancomycin trough concentration of the VA-AKI group was higher than that of the non-AKI group (20.50 ± 8.65 vs. 17.81 ± 9.51, p < 0.001). The length of stay of the VA-AKI group was significantly longer than that of the non-AKI group (p < 0.001). Patients with VA-AKI were more likely to have higher SOFA scores and lower GCS scores, which suggested that the illness of patients in the AKI group was more severe than that of patients in the non-AKI group (p < 0.001). Among the non-AKI group, 45 patients (6.09%) were exposed to other nephrotoxic medications, whereas in the AKI group, 712 patients (29.59%) were exposed to such medications(p < 0.001). The 30-day mortality in the AKI group was higher than that in the non-AKI group (23.4% vs. 10.1%, p < 0.001).
To further explore the association between troughs and VA-AKI, we compared concentrations in patients with different stages of acute kidney injury. As shown in Fig. 2, we did not find significant differences in stage 1, stage 2, and stage 3 (20.66 ± 10.17, 20.51 ± 8.00, 20.43 ± 8.36, p > 0.05).
Univariate and multivariate analyses
Logistic regression analysis showed the possible risk factors and protective factors associated with VA-AKI (Table 3). Both univariate and multivariable logistic models showed that VTC, comorbidities including congestive heart failure, and laboratory indicators including platelet, baseline creatinine, and albumin, SOFA, other nephrotoxic medications, and GCS score were all related to the development of VA-AKI. For each 1 mg/l increase in VTC, the odds ratio (OR) for VA-AKI increased by 2.5% (OR = 1.025, 95% confidence interval (CI) = 1.010–1.040, p = 0.001). The risk factors associated with 30-day mortality include age, congestive heart failure, cerebrovascular disease, malignant cancer, white blood cell count, bilirubin levels, albumin levels, glucose levels, VTC, GCS score, and SOFA score(Table 4). Specifically, the VTC was found to have an OR of 1.018 with a 95% CI of 1.006 to 1.031, indicating a statistically significant association with 30-day mortality (P < 0.001).
Restricted cubic splines
The identified risk factors of VA-AKI and 30-day mortality above were taken into account as covariates during the RCS analysis to investigate the linear dose–response relationship between the VTC and the outcomes, as illustrated in Fig. 3. The RCS model showed a linear dose‒response association between the vancomycin trough concentration and outcomes, including VA-AKI and 30-day mortality (Fig. 3). In Fig. 3A, the P value for nonlinearity was 0.08, and the P value for nonlinearity was 0.06 in Fig. 3B. In this study, the RCS curves revealed that VTC ranged of 19.67 to 35.72 mg/l for AKI and 19.17 to 42.86 mg/l for 30-day mortality exhibit OR with 95% CI above 1.
Kaplan‒Meier analysis
A significant difference was observed between patients with trough concentrations ≤ 20 and those with trough concentrations > 20 for all-cause 30-day mortality (10.1% vs. 23.4%, Fig. 4).
Subgroup analyses
Subgroup analyses were performed for sex (male or female), age (< 75 or ≥ 75 years), race (white, black/African American or other), SOFA score (< 6 or ≥ 6), shock (with or without), baseline creatinine (creatinine ≤ 1.2 µg/ml or creatinine > 1.2 µg/ml) and exposed to other nephrotoxic medications (yes or not). As shown in Fig. 5, VTC was still a risk factor in white people(OR 1.024,95%CI1.007,1.041), female patients(OR 1.041,95% CI 1.018–1.064), patients without shock(OR 1.026,95% CI 1.007–1.045), patients with SOFA > 6(OR 1.026,95%CI1.009–1.044) and patients with baseline creatinine > 1.2 mg/dl(OR 1.040,95% CI 1.014–1.066). In both the age < 75 and age ≥ 75 groups, the risks of VA-AKI increased with trough concentration (OR 1.021, 95% CI 1.002–1.041, OR 1.028, 95% CI 1.005–1.052, respectively). In with the exposed to other nephrotoxic medications and without exposed to other nephrotoxic medications, the risks of VA-AKI increased with trough concentration (OR 1.09, 95% CI 1.02–1.16, OR 1.02, 95% CI 1.01–1.04, respectively).
Discussion
In this study, we found that the incidence of VA-AKI in the elderly population was 76.5%, which was significantly higher than that in non-elderly patients. We found a 64.0% rate of VA-AKI if the trough was < 10 mg/l compared to rates of 72.5% for troughs of 10–15 mg/l, 73.7% for 15–20 mg/l, and 82.6% for > 20 mg/l (P < 0.05). Furthermore, for each mg/l increase, the OR for VA-AKI increased by 2.5%. A linear relationship between trough levels and VA-AKI levels was also observed in this study.
Logistic regression analysis revealed VTC was significantly associated with VA-AKI and 30-day mortality.
The exact pathophysiological mechanisms of VA-AKI are not yet fully understood. Oxidative stress is considered the primary mechanism of VA-AKI. When intracellular drug concentrations in the renal tubules of patients with risk factors are high, oxidative stress can lead to an imbalance between reactive oxygen species and antioxidants, altered mitochondrial function, and cellular apoptosis in proximal renal tubules. Vancomycin-associated renal tubular cast and allergic reaction are other possible mechanisms involved in VA-AKI [10]. Significant eosinophil infiltration was found in VA-AKI patients' kidney biopsy results [19]. It is proposed to be associated with the T-cell-mediated type-4 delayed hypersensitivity reaction or probable complement system activation [20, 21].
Due to narrow therapeutic index of vancomycin, careful dosing and monitoring are critical to ensure therapeutic efficacy while minimizing the risk of adverse events such as nephrotoxicity. Monitoring VTC allows for dosage adjustments to maintain concentrations within a range that is effective yet below nephrotoxic levels. This is particularly important in vulnerable populations, such as the elderly and those with pre-existing renal impairment. The recognition of VTC as a critical factor in vancomycin therapy aligns with existing clinical guidelines. Emerging evidence suggests that monitoring the area under the concentration–time curve (AUC) to minimum inhibitory concentration (MIC) ratio may be superior to trough concentration monitoring alone for predicting vancomycin efficacy and toxicity [22]. However, AUC monitoring is not yet universally available or feasible in all settings, making VTC monitoring a vital and more accessible tool for guiding vancomycin therapy.
Even if the pathophysiological mechanisms are unclear, the incidence of VA-AKI remains high. Previous studies have shown that almost 5%–43% of patients exposed to vancomycin will develop VA-AKI, which VA-AKI is more common than renal failure caused by other antibiotics [11, 23, 24]. Elderly people are at high risk of VA-AKI. A retrospective study enrolled elderly patients and was conducted in China [25]. Among 647 patients, 102 patients had confirmed VA-AKI, with an incidence of 15.8%, which differed widely from ours. Potential reasons are as follows: first, patients enrolled in our study were older. The mean age of the patients in this study was 76 years, and the mean age in the previous study was 71 years; Second, the elderly adults included are mainly white people. In our subgroup analysis, we did not find that trough concentration was an independent risk factor for VA-AKI in nonwhite people; Third, patients in our study were from intensive care units and emergency departments. We had a higher proportion of patients complicated with shock (44% vs. 11.6%). This means that the patients included in our study were in worse health status and had more serious illnesses. The severity of infection and ICU residence impact the development of AKI in patients exposed to vancomycin [26].
Numerous studies have recognized the relationship between trough levels and VA-AKI [8, 27,28,29]. A previous meta-analysis indicated that a cutoff of 15 mg/L detected VA-AKI with a sensitivity of 62.6% and a specificity of 65.5%, while applying a 20 mg/L threshold resulted in a sensitivity of 42.9% and a specificity of 82.5% [30]. Horey et al. found nephrotoxicity rates of 5%, 3%, 11%, 24%, and 82% for maximal troughs of 5–10 mg/L, 10.1–15, 15.1–20, 20.1–35, and > 35, respectively [31]. Similarly, Kassem et al. found that the incidences of AKI in vancomycin trough < 10, 10 ≤ vancomycin trough < 15, 15 ≤ vancomycin trough < 20, and vancomycin trough ≥ 20 mg/L subgroups were 8.02, 13.61, 13.70 and 31.82%, respectively [32]. Guillaume et al. found that a serum vancomycin level greater than 40 mg/L (OR 3.75; 95% CI, 1.40–10.37) was associated with VA-AKI. In another study, vancomycin nephrotoxicity occurred in 42 patients (29.6%) with trough concentrations > 15 mg/ml and 13 (8.9%) with trough concentrations of < 15 mg/ml. Multivariate regression analysis also showed that vancomycin trough concentrations of > 15 mg/ml were a risk factor for nephrotoxicity [33]. Previous studies also highlighted the need for careful vancomycin dosing to balance treatment efficacy and nephrotoxicity risk in enterococcal infections [34]. An adult trough concentration ≥ 15 mg/L improves outcomes but increases nephrotoxicity, while an AUC/MIC ratio ≥ 400 mg·h/L aligns with better responses [35]. Pediatric dosing requires lower trough levels compared to adults, emphasizing age-specific monitoring and adjustments to optimize therapeutic effectiveness and minimize renal harm [36].
There is no consensus on the exact threshold of renal injury caused by vancomycin trough concentrations, as this threshold depends on a variety of factors, such as the patient's age. For elderly patients (aged ≥ 80 years), a previous study also indicated that vancomycin trough concentrations ≥ 15 mg/L were independent risk factors leading to nephrotoxicity [37]. Moreover, vancomycin trough concentrations (initial or maximal trough concentration) may be different in different articles about VA-AKI. Some researchers only considered the initial trough level. However, vancomycin therapy should be guided by TDM, and the initial trough concentration may differ widely from subsequent trough levels. Since vancomycin is mainly excreted through the kidney, reduced kidney function from any cause will lead to an elevated trough level. The risk of VA-AKI increases with trough concentrations. In our opinion, the maximal trough level was more appropriate. In this study, we found that the risks of AKI significantly increased when the trough concentration was > 19.67 mg/L. In contrast, if the trough concentration was < 7.8, the risk of VA-AKI did not increase with the trough concentration. We observed significant differences in trough concentration between non-AKI patients and AKI patients (17.81 ± 9.51 vs. 20.50 ± 8.65, p < 0.001) but no differences in trough concentration among different AKI-stage patients.
In addition to vancomycin concentration, congestive heart failure was an independent risk factor for VA-AKI (OR 2.254, 95% CI 1.323–3.840). However, we did not find an association between renal diseases and VA-AKI (OR 0.905, 95% CI 0.638–1.284). Instead, baseline creatinine was closely related to subsequent VA-AKI. It is suggested that patients with kidney disease that leads to renal dysfunction are susceptible to VA-AKI. To reduce the effect of other confounding factors such as exposure to other nephrotoxic drugs, age, and disease severity, we performed a multifactorial regression analysis and found risk factors for AKI including VTC. The severity of illness and infection are well-known risk factors for VA-AKI. In our study, SOFA and shock were proven to be risk factors for VA-AKI (OR 1.208,95% CI 1.142–1.279; OR 1.025, 95% CI 1.010–1.040, respectively). The identified risk factors of VA-AKI and 30-day mortality above were taken into account as covariates during the RCS analysis and an linear dose–response relationship between the VTC and the outcomes was observed.
Similar to Pan’s study [25], the serum albumin level was also observed as an independent protective factor. The protective effects of albumin are alleviating oxygen stress and oxidative damage and binding or delivering protective lysophosphatidic acid [38, 39]. Another laboratory test, platelet count, was also associated with VA-AKI. The potential mechanism may be impaired renal microvascular circulation due to a decreased blood flow rate by microthrombi [40, 41].
The relationship between trough concentration and 30-day mortality was also explored. We found that the trough level was associated with 30-day mortality. When the trough concentration is > 19.17 mg/L, the risk of 30-day mortality will significantly increase. Increasing concentrations of vancomycin cannot enhance the ability of bacterial killing but significantly increase the risks of VA-AKI [42]. In this study, we found that increasing concentrations may also increase the risk of 30-day mortality.
In conclusion, this study found the significant association between VTC and the incidence of VA-AKI and 30-day mortality in critically ill elderly adults. The RCS curves indicated concentration ranges for AKI (19.67–35.72 mg/L) and 30-day mortality (19.17–42.86 mg/L), signifying increased risk.
There were some limitations in this study. First:retrospective study design. The study employed a retrospective design, which may introduce information bias and missing data. Data extracted from medical information databases could be subject to issues of data quality and accuracy. Second: data source. The study utilized data from the Medical Information Mart-IV (MIMIC-IV) and eICU databases, which may represent specific patient populations. Generalizability of the findings might be limited to other settings or patient cohorts. Third: potential confounding factors. Even though we used multiple regression analyses, retrospective studies may not account for all potential confounding factors that could influence the occurrence of VA-AKI and the study outcomes. Forth: sample size. Although the study includes a substantial number of critically ill elderly patients, the sample size may still be limited for some subgroup analyses. Fifth, we did not investigate the relationship between the vancomycin regimen and VA-AKI. Sixth: microbiological outcomes: Our study did not extensively explore microbiological outcomes, such as the rate of eradication of specific pathogens and the development of resistance. Seventh:Prospective Validation: The findings from our study should be validated in prospective studies to confirm the identified risk factors and associations with clinical outcomes. Finally:long-term outcomes: Our study focused on short-term outcomes, specifically VA-AKI and 30-day mortality. Further studies could investigate the long-term consequences of vancomycin-associated AKI in the elderly.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Petejova N, Martinek A, Zadrazil J, Kanova M, Klementa V, Sigutova R, Kacirova I, Hrabovsky V, Svagera Z, Stejskal D. Acute kidney injury in septic patients treated by selected nephrotoxic antibiotic agents-pathophysiology and biomarkers-a review. Int J Mol Sci. 2020;21(19):7115.
Chastre J, Blasi F, Masterton RG, Rello J, Torres A, Welte T. European perspective and update on the management of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect. 2014;20(Suppl 4):19–36.
Mensa J, Soriano A, Llinares P, Barberan J, Montejo M, Salavert M, Alvarez-Rocha L, Maseda E, Moreno A, Pasquau J, et al. Guidelines for antimicrobial treatment of the infection by Staphylococcus aureus. Rev Esp Quimioter. 2013;26(Suppl 1):1–84.
Ye ZK, Chen YL, Chen K, Zhang XL, Du GH, He B, Li DK, Liu YN, Yang KH, Zhang YY, et al. Therapeutic drug monitoring of vancomycin: a guideline of the division of therapeutic drug monitoring, Chinese Pharmacological Society. J Antimicrob Chemother. 2016;71(11):3020–5.
Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325–7.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, Mueller BA, Pai MP, Wong-Beringer A, Rotschafer JC, et al. Executive Summary: Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2020;40(4):363–7.
Abdelmessih E, Patel N, Vekaria J, Crovetto B, SanFilippo S, Adams C, Brunetti L. Vancomycin area under the curve versus trough only guided dosing and the risk of acute kidney injury: Systematic review and meta-analysis. Pharmacotherapy. 2022;42(9):741–53.
Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, Ford KD, Zervos MJ, Ramirez JA, Kett DH, et al. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther. 2012;34(1):149–57.
Morales-Alvarez MC. Nephrotoxicity of Antimicrobials and Antibiotics. Adv Chronic Kidney Dis. 2020;27(1):31–7.
Kan WC, Chen YC, Wu VC, Shiao CC. Vancomycin-associated acute kidney injury: a narrative review from pathophysiology to clinical application. Int J Mol Sci. 2022;23(4):2052.
Jorgensen SCJ, Murray KP, Lagnf AM, Melvin S, Bhatia S, Shamim MD, Smith JR, Brade KD, Simon SP, Nagel J, et al. A multicenter evaluation of vancomycin-associated acute kidney injury in hospitalized patients with acute bacterial skin and skin structure infections. Infect Dis Ther. 2020;9(1):89–106.
Liu F, Chen J, Li Z, Meng X. Recent advances in epigenetics of age-related kidney diseases. Genes (Basel). 2022;13(5):796.
Laou E, Mavridis T, Papagiannakis N, Pais G, Chighine A, Chang J, Locci E, D’Aloja E, Scheetz M, Chalkias A, et al. Blood biomarkers and metabolomic profiling for the early diagnosis of vancomycin-associated acute kidney injury: a systematic review and meta-analysis of experimental studies. J Pers Med. 2022;12(9):1397.
Daste A, Domblides C, Gross-Goupil M, Chakiba C, Quivy A, Cochin V, de Mones E, Larmonier N, Soubeyran P, Ravaud A. Immune checkpoint inhibitors and elderly people: a review. Eur J Cancer. 2017;82:155–66.
Montero N, Perez-Saez MJ, Pascual J, Group DW, Board DEE, Abramowicz D, Budde K, Dudley C, Hazzan M, Klinger M, et al. Immunosuppression in the elderly renal allograft recipient: a systematic review. Transplant Rev. 2016;30(3):144–53.
Yang J, Li Y, Liu Q, Li L, Feng A, Wang T, Zheng S, Xu A, Lyu J. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. 2020;13(1):57–69.
Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215-220.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-184.
Tantranont N, Obi C, Luque Y, Truong LD. Vancomycin nephrotoxicity: Vancomycin tubular casts with characteristic electron microscopic findings. Clin Nephrol Case Stud. 2019;7:66–72.
Htike NL, Santoro J, Gilbert B, Elfenbein IB, Teehan G. Biopsy-proven vancomycin-associated interstitial nephritis and acute tubular necrosis. Clin Exp Nephrol. 2012;16(2):320–4.
Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med. 2008;36(4 Suppl):S216-223.
Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58(1):309–16.
Awdishu L, Le A, Amato J, Jani V, Bal S, Mills RH, Carrillo-Terrazas M, Gonzalez DJ, Tolwani A, Acharya A, et al. Urinary exosomes identify inflammatory pathways in vancomycin associated acute kidney injury. Int J Mol Sci. 2021;22(6):784.
Sinha Ray A, Haikal A, Hammoud KA, Yu AS. Vancomycin and the risk of AKI: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2016;11(12):2132–40.
Pan KM, Wu Y, Chen C, Chen ZZ, Xu JA, Cao L, Xu Q, Wu W, Dai PF, Li XY, et al. Vancomycin-induced acute kidney injury in elderly Chinese patients: a single-centre cross-sectional study. Br J Clin Pharmacol. 2018;84(8):1706–18.
Filippone EJ, Kraft WK, Farber JL. The Nephrotoxicity of Vancomycin. Clin Pharmacol Ther. 2017;102(3):459–69.
Barriere SL, Stryjewski ME, Corey GR, Genter FC, Rubinstein E. Effect of vancomycin serum trough levels on outcomes in patients with nosocomial pneumonia due to Staphylococcus aureus: a retrospective, post hoc, subgroup analysis of the Phase 3 ATTAIN studies. BMC Infect Dis. 2014;14:183.
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–9.
Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49(4):507–14.
Bellos I, Daskalakis G, Pergialiotis V. Relationship of vancomycin trough levels with acute kidney injury risk: an exposure-toxicity meta-analysis. J Antimicrob Chemother. 2020;75(10):2725–34.
Horey A, Mergenhagen KA, Mattappallil A. The Relationship of nephrotoxicity to vancomycin trough serum concentrations in a veteran’s population: a retrospective analysis. Ann Pharmacother. 2012;46(11):1477–83.
Hammoud K, Brimacombe M, Yu A, Goodloe N, Haidar W, El Atrouni W. Vancomycin trough and acute kidney injury: a large retrospective, cohort study. Am J Nephrol. 2016;44(6):456–61.
Bosso JA, Nappi J, Rudisill C, Wellein M, Bookstaver PB, Swindler J, Mauldin PD. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother. 2011;55(12):5475–9.
Katip W, Okonogi S, Oberdorfer P. The thirty-day mortality rate and nephrotoxicity associated with trough serum vancomycin concentrations during treatment of Enterococcal infections: a propensity score matching analysis. Front Pharmacol. 2021;12:773994.
Katip W, Oberdorfer P. A monocentric retrospective study of AUC/MIC Ratio of Vancomycin Associated with Clinical Outcomes and Nephrotoxicity in Patients with Enterococcal Infections. Pharmaceutics. 2021;13(9):1378.
Issaranggoon Na Ayuthaya S, Katip W, Oberdorfer P, Lucksiri A. Correlation of the vancomycin 24-h area under the concentration-time curve (AUC(24)) and trough serum concentration in children with severe infection: a clinical pharmacokinetic study. Int J Infect Dis. 2020;92:151–9.
Huang M, Wu H, Zhou J, Xu M, Zhou S. Efficacy of vancomycin on gram-positive bacterial infection in elderly critical patients and risk factors associated with nephrotoxicity. Arch Iran Med. 2018;21(8):349–55.
Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34(7):670–6.
Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. 2010;36(10):1657–65.
Hui S, Yu C. Platelet counts, acute kidney injury, and mortality after coronary artery bypass grafting surgery. Anesthesiology. 2016;125(2):437–8.
Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30(8):1765–71.
Larsson AJ, Walker KJ, Raddatz JK, Rotschafer JC. The concentration-independent effect of monoexponential and biexponential decay in vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J Antimicrob Chemother. 1996;38(4):589–97.
Acknowledgements
Not applicable
Funding
No funding.
Author information
Authors and Affiliations
Contributions
L,C, J L acquired the data,drafted and revised the manuscript. JZ W and Y J and YM L designed the study, provided supervision and critically revised the manuscript. All authors approve the final version of the manuscript and agree to be accountable for all aspects of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This project was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA, USA) and the Massachusetts Institute of Technology (Cambridge, MA, USA). The study authors had complete the CITI Program course (Human Research and Data or Specimens Only Research) to gain access to the database (Record ID: 47755805). The information of patients in this study is deidentified and anonymous.Individual permission was not required for this retrospective analysis. And ethical review and informed consent were waived. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, J., Lin, J., Weng, J. et al. Association between trough serum vancomycin concentration and vancomycin-associated acute kidney injury and 30-day mortality in critically ill elderly adults. BMC Infect Dis 24, 330 (2024). https://doi.org/10.1186/s12879-024-09227-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09227-x