Abstract
Background
Aspergillus spp liver abscess is a relatively rare entity and thus far no systematic review has been performed examining patients’ demographics, clinical manifestations, diagnosis, management, and outcome.
Methods
We performed a systematic review of the literature using MEDLINE and LILACS databases. We searched for articles published in the period from January 1990 to December 24, 2022, to identify patients who developed liver abscesses due to Aspergillus spp.
Results
Our search yielded 21 patients all of whom had invasive aspergillosis confirmed on liver biopsy. Of these patients 81% were adults, and 60% were males. The majority (86%) of patients were immunocompromised and 95% had symptomatic disease at the time of diagnosis. The most common symptoms were fever (79%), abdominal pain (47%), and constitutional symptoms (weight loss, chills, night sweats, fatigue) (38%). Liver enzymes were elevated in 50%, serum galactomannan was positive in 57%, and fungal blood cultures were positive in only 11%. Co-infection with other pathogens preceded development of apsergillosis in one-third of patients, and the majority of the abscesses (43%) were cryptogenic. In the remaining patients with known source, 28% of patients developed liver abscess through dissemination from the lungs, 19% through the portal vein system, and in 10% liver abscess developed through contiguous spread. The most common imaging modality was abdominal computerized tomography done in 86% of patients. Solitary abscess was present in 52% of patients while 48% had multiple abscesses. Inadequate initial empiric therapy was prescribed in 60% of patients and in 44% of patients definite treatment included combination therapy with two or more antifungal agents. Percutaneous drainage of the abscesses was done in 40% of patients, while 20% required liver resection for the treatment of the abscess. Overall mortality was very high at 38%.
Conclusion
Further studies are urgently needed for a better understanding of pathophysiology of liver aspergillosis and for developement of newer blood markers in order to expedite diagnosis and decrease mortality.
Similar content being viewed by others
Background
A liver abscess (LA) is a rare condition characterized by the formation of a purulent cavity by microorganisms in the liver [1,2,3,4,5]. LA is classified as bacterial, protozoan (amoebic), or fungal [1,2,3,4,5]. The most common causes of bacterial (pyogenic) liver abscesses (BLA) are Escherichia coli, Klebsiella spp, Streptococcus anginosus group, Staphylococcus aureus, and anaerobes. Amebic liver abscesses (ALA) are the most common manifestation of extra-intestinal amebiasis caused by Entamoeba histolytica.Fungal pathogens, however, are comparitively rare cause of LA [1,2,3,4,5].
The incidence of BLA varies by geography. For example, in the North American population the incidence is around 2 per 100,000, but 17.6 per 100,000 inhabitants in Taiwan. Meanwhile in Sweden, the incidence of BLA has increased almost three fold over the last decade, from 1.8/100,000 person-years in 2011 to 5.2/100,000 person-years in 2020 which is partially explained by their aging population [6].
Fungal infections in the liver are less common compared to BLA. Candida spp and Aspergillus spp are the most common causative agents in patients with hematological malignancy [7,8,9,10,11,12]. The incidence of these infections has decreased due to the use of antifungal prophylaxis, particularly amongst patients with cancer on immunosuppressive therapy. The most common Aspergillus spp causing infections in humans are: A. fumigatus, A. flavus, A. niger, and A. terreus [7, 8]. The most common and most severe form of aspergillosis is invasive aspergillosis (IA), followed by a chronic, allergic form termed chronic bronchopulmonary aspergillosis [9,10,11, 13]. While the lungs and upper respiratory organs are most commonly affected in aspergillosis, other visceral organs are typcially infected following dissemination from the primary lung focus [9, 10].
Aspergillus spp liver abscess or invasive liver aspergillosis (ILA) is a rare extrapulmonary manifestation of IA [14]. In a recent autopsy study, the liver was amongst the least commonly affected extrapulmonary sites, and hepatic aspergillosis was found in only 10% of patients with fatal IA compared to central nervous system and cardiac IA that were the most common extrapulmonary sites involved, in 24% of cases each [15]. There is a paucity of evidence about patients’ characteristics, risk factors, management, and outcomes of patients with ILA. The data we have about this rare clinical manifestation of IA are based on case reports, case series, and expert opinions. Due to the rarity of the disease, there are no retrospective or prospective studies on this specific topic. Therefore, we performed a scoping review of case reports and case series to understand more about ILA and discuss our findings in the context of established knowledge on the more common etiologies of liver abscesses(i.e.,BLA and ALA.)
Methods
A scoping review of the literature was performed by searching the MEDLINE database via PubMed search engine and Latin American and Caribbean Health Sciences Literature (LILACS) database via Bvsalud search engine from January 1990 to December 24, 2022, in order to identify patients who developed liver abscess due to Aspergillus spp using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping review (ScR) methodology. The keywords used for the literature search were: “aspergillus or aspergillosis”, “liver or hepatic”, and “infection or abscess”. Furthermore, the reference list of identified articles was manually screened to identify additional cases that can be included in our analysis.
Two authors (I.D. and E.M.) independently and blindly screened the titles, abstracts, and full manuscripts of the identified articles reporting cases of microbiologically proven ILA. Articles that were not case reports and did not report aspergillosis, articles written in a language other than English and Portuguese, and articles that did not contain sufficient information were excluded. Any discrepancies or uncertainties were resolved by the first author (I.D.).
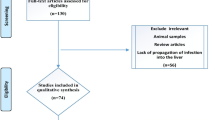
A total of 1213 articles were identified by the initial search, out of which 14 were duplicates.. The selection process resulted in a total of 21 articles in this study [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. A detailed PRISMA flowchart is illustrated in Fig. 1.
After the final articles were selected, data was collected and the following variables were extracted: age, gender, comorbidities/immunosuppression (high dose steroids, concurrent or recent chemotherapy in the past 1 year, solid organ transplant [SOT] or hematopoietic stem cell transplant [HSCT], hematologic malignancy - not achieved remission, Acquired Immunodeficiency Syndrome [AIDS], use of Tumor Necrosis Factor [TNF] alpha or anti-CD20 therapy in past 2 years, diabetes mellitus [DM]), presence of any other infections, primary Aspergillus focus and portal of entry, symptoms of abdominal pain, fever, or constitutional symptoms (weight loss, chills, night sweats, fatigue), laboratory findings (liver function tests [LFTs], serum galactomannan [GM], liver biopsy, cultures), imaging, management, and outcome.
All cases included met criteria for definite IA by liver biopsy specimen either demonstrating fungal hyphae and/or fungal cultures growing Aspergillus spp. [10, 36].
Results
Demographic characteristics
The total number of cases identified in this scoping review was 21 patients, out of which 17 were adults. The mean age was 38 ± 19 years. Out of 4 pediatric cases, two were males and one was female, while sex was not reported in one child. Immunosuppression was present in 86% of these patients; most commonly due to chemotherapy and hematological malignancy – not achieved remission (35%), followed by organ transplants (20%), while other immune-compromising conditions such as AIDS, aplastic anemia, and splenectomy were less common. For the pediatric group, 3 out of 4 had inherited immunodeficiencies (Table 1).
Source of infection and coinfection
The abscesses were cryptogenic in 43% of cases. A presumed pulmonary source of infection with secondary liver involvement by hematogenous dissemination was documented in 28% of cases. Liver infection by dissemination from the gastrointestinal tract via the portal vein system was documented in in 19%. In the remaining 10%, spread to liver occured contiguously through the skin from right-sided rib osteomyelitis and from partially treated left adrenal gland aspergillosis. Co-infection with another pathogen was present in 33% of cases.
Clinical presentation
Symptomatic disease was present in 95% of cases, while in a single case (5%), the disease was asymptomatic and discovered by serial monitoring of galactomannan test in a post-HSCT patient. The most commonly reported symptoms included fever, abdominal pain, and constitutional symptoms in 79%, 47%, and 38% of patients, respectively (Table 1).
Laboratory findings
All 21 patients had a liver biopsy with Aspergillus spp demonstrated from the liver tissue that met the criteria for confirmed invasive aspergillosis. There was a high variability of reported laboratory findings and many case reports had not reported their full spectrum of laboratory investigation. Furthermore, as many patients were on chemotherapy or in the post-transplant period, complete blood cell counts were frequently affected by the primary problem. LFTs were reported in 14 cases and of those, 50% cases reported liver enzyme elevation. Serum GM and blood cultures were not reported in many cases. In those who reported it, serum GM was positive in 57%. Fungal blood cultures for Aspergillus were positive in only 1 of 9 patients (positivity rate of 11%).
Imaging
The most common imaging modality was abdominal computerized tomography (CT) performed in 81% of patients at any point during the management.Abdominal ultrasound (US), as an initial test, was performed in 42% of patients. Abdominal magnetic resonance imaging (MRI) was done in only one patient. Abscesses were solitary in 52% of patients, while multiple abscesses were reported in 48%. The abscess size was variable and ranged from 2.2 cm to 9 cm in diameter.
Management
Initial empiric antimicrobial treatment was inadequate in 60% of patients where a bacterial pathogen was suspected and Aspergillus-specific antimicrobial treatment was given only after the cultures were reported. In 53% of patients, monotherapy with either amphotericin, itraconazole, voriconazole, or caspofungin was utilized, while in the remaining 47% combination therapy with any of the two agents was used.
Outcome
Overall mortality was substantial and death due to complications from overwhelming infection or related complications occurred in 8 out of 21 patients (38%), prior to the diagnosis being established or during the treatment course.
Discussion
Demographics, comorbidities and the risk factors
Similar to BLA and ALA, ILA occurred more frequently in men. It remains unclear why liver abscesses are more common in men, but across the studies that seems to be a consistent finding regardless of the etiology of liver abscess [2, 4, 5]. In patients with ALA, this noticeable discrepancy could be explained by several mechanisms, including the effect of testosterone, and by alcohol consumption which is traditionally more prominent in men. In cases of E. histolytica infection, alcohol consumption could contribute to higher infection rates since it has been hypothesized that locally produced alcoholic drinks in endemic regions (e.g., palm tree wine) can contain a significant amount of E. histolytica [2, 37].
A recent study from Sweden demonstrated that BLA incidence has increased over the last decade, and this was attributed to their aging population [6]. Older age is considered a risk factor for BLA but not for ILA. In our review, the mean age was 38 years which is much younger than 65 years as reported in previous studies on BLA, yet similar to the mean age of patients with ALA of 41 years [2, 4, 5, 38]. The reason why ILA is a disease of a younger population is probably related to the predispostion of lymphoma and leukemia for this popluation, and organ transplant recipients tend to be younger.
The risk factors for BLA, ALA, and ILA are vastly different (Table 2). The most recognized risk factors for the development of BLA are advanced age, uncontrolled DM, liver trauma, intraabdominal surgery, use of proton pump inhibitors (PPI), and biliary pathology [2, 4, 5, 39]. The most important risk factors for amebic liver abscesses (ALA) are poor sanitation, travel to endemic areas, and malnutrition [2] . In contrast, patients with fungal liver abscess are usually profoundly immunocompromised as evidenced by these review findings. The risk factor for hepatosplenic candidiasis, including liver abscess, is prolonged and severe neutropenia [40]. Only 3 patients in our review were immunocompetent. In these patients, aspergillosis developed during complications of postpartum necrotizing fascitis [20],as a consequence of gastric ulcer perforation which communicated with the left liver lobe [28], or from contiguous spread from adrenal gland aspergillosis [41]. All other patients (86%) were in an immunocompromised state: either HSCT or SOT recipients, or patients who were undergoing high intensity chemotherapy for hematologic malignancies (leukemia and lymphoma). Prolonged neutropenia is a strong risk factor for Aspergillus spp infection in patients with hematologic malignancies [42]. Children who developed ILA typically had a profound inherited immunodeficiency in the form of chronic granulomatous disease (CGD), purine nucleoside phosphorylase deficiency (PNP), or common variable immunodeficiency (CVID) [22, 23, 29].
Other recently described risk factors for IA include intensive care unit (ICU) stay and preceding bacterial or viral infection [43,44,45]. In this review, 33% of patients had co-infection with another pathogen around the time of liver aspergillosis diagnosis. These co-infections are likely additional risk factors which contribute to development of IA through epithelial damage, allowing for easier dissemination of aspergillosis. In fact, up to 60% of patients diagnosed with invasive pulmonary aspergillosis in an autopsy series were found to have co-infection with another pathogen [15], however in this review it was 33%.
Pathogenesis of liver aspergillosis
Aspergillus is a ubiquitous environmental fungus. While it is a harmless colonizer for the majority of immunocompetent people, it is an opportunistic pathogen in people with defects in cellular and/or humoral immunity. Most IA involves the lungs with inhalation of spores as the most common portal of entry, whereas the visceral organs are usually affected by hematogenous dissemination. Following the lungs, the most common affected organs are the paranasal sinuses and the brain [11, 13, 46]. The liver is very rarely affected in aspergillosis, although some autopsy reports suggest that gastrointestinal (including liver) aspergillosis might be underreported [17, 47].
Of the 21 patients with ILA described here, 28% had disease due to hematogenous seeding from the infectious foci in the lungs, while in 19% of cases the portal of entry was presumed to be the gastrointestinal tract. A gastrointestinal portal of entry has been hypothesized to occur due to profound neutropenia, mucositis and damage to the intestinal epithelium during chemotherapy which allows the fungus to migrate and seed into the liver [17] or due to disruption of intestinal barries during intraabdominal surgery as illustrated in remaining 3 cases [16, 20, 28]. In these cases patients had abdominal surgery for various reasons (perforated peptic ulcer, necrotizing facitis following Cesarean section, and liver transplant) and developed liver abscess 2–4 weeks after that. Cryptogenic liver abscesses were the most commonly documented in 43% in cases where no portal of entry could be identified. Given its angioinvasive features, it is not surprising that the majority of extrapulmonary aspergillosis results from hematogenous dissemination. In two patients the infection occured through contiguous spread from the rib osteomyelitis and from left adrenal gland aspergilosis [41].
Clinical characteristics and laboratory analysis
Patients with BLA usually present with fever, leukocytosis, and abdominal pain (Table 2). Patients with ALA are more likely to present with nausea, diarrhea, and protracted constitutional symptoms [48]. Patients with ILA, however, may present differently. Due to profound immunosuppression, these patients are less likely to mount a leukocytosis [49]. Fever, however, remains the most common, and sometimes the only, sign of the infection.This is particularly true in patients with prolonged and severe neutropenia (absolute neutrophil count of less than 0.5 × 109/L (< 500/μL),). Although less common than in patients with BLA, we found fever to be the most common sign of infection in patients with ILA, occuring in 79%. Abdominal pain was present in 47% of cases and we found constitutional symptoms to be present in 38%. Of note, in some immunocompetent patients the liver can be affected diffusely by a disseminated form of aspergillosis with morphologic features of granuloma rather than a well-formed abscess [50]. Liver enzymes were reported in 50% of the 14 cases that reported this information, and all of these patients had mild to moderate elevation in transaminases with hepatocellular pattern of liver injury. Cholestatic pattern of liver injury was not observed in any of the patients who reported the liver function test and one patient had mixed patter of liver injury.
Diagnosis
A definite diagnosis of IA is established by demonstrating fungal hyphae in tissue specimens or by demonstrating Aspergillus spp. growth in fungal cultures of liver tissue. In this review, we included only case reports and case series that fulfilled this definition. Other laboratory (serum GM, beta D glucan, fungal blood cultures) and imaging findings might assist in establishing diagnosis but are not sufficient [10, 51].
The reported sensitivity of serum GM antigen index can vary from 30 to 100%, and specifity is generally reported as > 75%. These values vary significantly between Aspergillus and non-Aspergillus fungi, testing assay, and host factors such as age and prior HSCT or SOT. While galactomannan is relased into serum from the cell wall of replicating Aspergillus species, it is also present in the cell wall of several other fungi and can cross react with antibiotics such as piperacillin-tazobactam and amoxicillin-clavulanate. A low yield from fungal blood cultures is true for other fungal pathogens too, for example Candida spp where sensitivity of blood cultures is limited, and up to 70% of patients with hepatosplenic candidiasis might not have documented fungemia [52, 53].
The findings from this report with regard to the serum GM test and fungal blood cultures must be interpreted with caution since many authors of these case reports had not reported the results of these tests. However, if we take into account only the cases that reported results, a positive serum GM was seen in 57% of patients, and fungal blood cultures were positive in 11%. This is in alignment with reports from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) [51] which state that the serum GM test is more sensitive than fungal blood culture for the diagnosis of invasive aspergillosis. In one case report from Italy, the diagnosis was challenging when the clinical and radiological findings mimicked hepatosplenic candidiasis. In this instance, the diagnosis was established initially by assessment of Aspergillus specific T cells by an enzyme-linked immunospot (ELISPOT) assay that demonstrated a high number of Aspergillus-specific T cells producing interleukin-10 [TH2(IL-10)] and a low number of Aspergillus-specific T cells producing gamma interferon [TH1(IFN-γ)] [33]. In this case, the diagnosis was later confirmed by demonstrating fungal growth in liver biopsy specimen and pathohistology.
While serology can be useful to establish a diagnosis in patients with ALA, for ILA and for BLA serologies do not have reliable diagnostic utility.
Imaging
For diagnosis of LA, abdominal CT and US are the most commonly utilized diagnostic tools, with high sensitivity (US: 85–95%; CT: 100%) [54]. ALA are greater in size and more commonly solitary compared to BLA which are more frequently multiple and bilobar [55]. In this review of ILA, 52% had a solitary abscess and 48% had multiple abscesses.
Management
The guidelines of the Infectious Diseases Society of America [10] recommend voriconazole as initial therapy for invasive aspergillosis based on randomized clinical trials that demonstrated voriconazole to be more efficient than amphotericin B deoxycholate in relation to survival and clinical improvement (71% vs 58%) [10]. In the current review, only 3 patients received voriconazole monotherapy, 5 recieved amphotericin monotherapy, and 8 received combination of 2 or more antifungal agents. Of 8 patients who received combination therapy, 5 patients received voriconazole plus echinocandin. Of these 5 patients, 2 died. Combination of voriconazole with echinocandin might provide mortality benefit in certain patient populations [56]. These discrepancies with IDSA guidelines are due to the majority of the older reports being published prior to 2016 when the latest IDSA guidelines on aspergilosis management were published.
Amongst the 21 patients described in this systematic review, surgical resection of the liver abscess was performed in 20%, while percutaneous drainage was adequate for source control in 40%. This highlights the challanges associated with treatment of liver aspergillosis and emphasizes that medical management alone is frequently insufficient. Whether or not percutaneous catheter drainage of liver abscess improves outcomes in patients with ILA is debatable. While it seems intuitive that faster source control by aspiration or surgical drainage would potentially lead to a better outcome, firm data are lacking. Moreover patients with ILA are often too ill to undergo any type of procedure.
Outcome
The mortality of liver abscesses as a whole has decreased dramatically over the years [2]. This improvement in morbidity and mortality is attributed to better and more available imaging techniques, and a larger armementarium of medication available to treat liver abscesses. In our review, the mortality of patients with ILA was 38% which was much higher compared to ALA and BLA (Table 2). This higher mortality is due to inherent risk factors in this patient population who tend to be much sicker, immunocompromised, and more challanging to diagnose. Additionally, in ILA patients, the diagnosis is often delayed and empiric therapy is frequently inadequate.
Limitations
While this study brings important data from summarizing previously published case reports on this rare entity it has notable limitations. First, publication bias is inevitable in this type of review, and we acknowledge this shortcoming. Second, the study sample is relatively small, and not all cases reported all variables of interest. Finally, some of the high-quality case reports might have been missed if they were not published in the journals indexed in the two databases we used, or if they were published in a language other than English or Portuguese.
Conclusions
Patients with liver abscess due to aspergilosis have very high overall mortality, 38%, which is higher than in those with bacterial or amoebic etiology. These patients are often immunocompromised and diagnosis is delayed due to low sensitivity and specificity of diagnostic tests. Further prospective studies are urgently needed to evaluate novel biomarkers that might expedite diagnosis and improve the outcome in this patient population.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Lardière-Deguelte S, et al. Hepatic abscess: diagnosis and management. J Visc Surg. 2015;152(4):231–43.
Roediger R, Lisker-Melman M. Pyogenic and amebic infections of the liver. Gastroenterol Clin N Am. 2020;49(2):361–77.
Khim G, et al. Liver abscess: diagnostic and management issues found in the low resource setting. Br Med Bull. 2019;132(1):45–52.
Serraino C, et al. Characteristics and management of pyogenic liver abscess: a European experience. Medicine (Baltimore). 2018;97(19):e0628.
Nie S, Lin D, Li X. Clinical characteristics and management of 106 patients with pyogenic liver abscess in a traditional Chinese hospital. Front Surg. 2022;9:1041746.
Svensson E, et al. Increasing incidence of pyogenic liver abscess in southern Sweden: a population-based study from 2011 to 2020. Infect Dis (Lond). 2023;55(6):375–83.
Gautier M, Normand AC, Ranque S. Previously unknown species of aspergillus. Clin Microbiol Infect. 2016;22(8):662–9.
Where Aspergillosis Comes From. 2021. Available from: https://www.cdc.gov/fungal/diseases/aspergillosis/causes.html.
Thompson GR. 3rd and J.H. Young, aspergillus infections. N Engl J Med. 2021;385(16):1496–509.
Patterson TF, et al. Practice guidelines for the diagnosis and Management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–e60.
Rudramurthy SM, et al. Invasive aspergillosis by aspergillus flavus: epidemiology, diagnosis Antifungal Resistance, and Management. J Fungi (Basel). 2019;5(3)
Ledoux M-P, Herbrecht R. Invasive Pulmonary Aspergillosis. J Fungi. 2023;9(2):131.
Ledoux MP, Herbrecht R. Invasive Pulmonary Aspergillosis. J Fungi (Basel). 2023;9:2.
López-Cortés LE, et al. Invasive aspergillosis with extrapulmonary involvement: pathogenesis, clinical characteristics and prognosis. Rev Iberoam Micol. 2012;29(3):139–43.
Mudrakola HV, et al. Autopsy study of fatal invasive pulmonary aspergillosis: often undiagnosed premortem. Respir Med. 2022;199:106882.
Jafarian A, Kasraianfard A, Nassiri-Toosi M. Revision liver transplant for persistent infection and localized aspergillosis after hepatic artery thrombosis. Exp Clin Transplant. 2014;12(4):381–3.
Chasan R, et al. Primary hepatic aspergillosis following induction chemotherapy for acute leukemia. Transpl Infect Dis. 2013;15(5):E201–5.
Gupta KL, et al. Progression of hepatic aspergillosis following second renal transplantation in a patient with recurrent glomerulonephritis. Indian J Pathol Microbiol. 2012;55(4):580–2.
Yamada R, et al. Successful treatment of aspergillus liver abscesses in a patient with acute monoblastic leukemia using combination antifungal therapy including micafungin as a key drug. Int J Hematol. 2010;91(4):711–5.
Rieder J, et al. Successful management of aspergillus liver abscess in a patient with necrotizing fasciitis. Dig Dis Sci. 2007;52(6):1548–53.
van der Velden WJ, et al. Primary hepatic invasive aspergillosis with progression after rituximab therapy for a post transplantation lymphoproliferative disorder. Ann Hematol. 2006;85(9):621–3.
Mamishi S, et al. A case of invasive aspergillosis in CGD patient successfully treated with amphotericin B and INF-gamma. Ann Clin Microbiol Antimicrob. 2005;4:4.
Trachana M, et al. Case report. Hepatic abscesses due to aspergillus terreus in an immunodeficient child. Mycoses. 2001;44(9–10):415–8.
Mazza D, et al. Survival of a liver graft recipient treated for an aspergillar liver abscess. Clin Infect Dis. 1996;23(4):831–2.
Yu L, Su M, Liu Q. Myelodysplastic syndrome with aspergillus fumigatus infection: a case report and literature review. Radiol Infect Diseases. 2017;4(1):26–8.
Scott CJ, et al. Invasive aspergillus fumigatus associated with liver and bone involvement in a patient with AIDS. Int J Infect Dis. 2007;11(6):550–3.
Lee TY, et al. Hepatic abscess caused by aspergillus fumigatus infection following splenectomy and immunosuppressive therapy. J Formos Med Assoc. 2003;102(7):501–5.
Vairani G, Rebeschini R, Barbazza R. Hepatic and subcutaneous abscesses due to aspergillosis. Initial diagnosis of a case by intraoperative fine needle aspiration cytology. Acta Cytol. 1990;34(6):891–4.
Aytekin C, et al. Purine nucleoside phosphorylase deficiency with fatal course in two sisters. Eur J Pediatr. 2010;169(3):311–4.
Poovorawan K, et al. Hepatic lymphoma and splenic aspergillosis mimicking HEPATOSPLENIC abscesses from MELIOIDOSIS in THAILAND. Southeast Asian J Trop Med Public Health. 2016;47(2):223–6.
Filice C, et al. Ultrasonographic and microbiological diagnosis of mycetic liver abscesses in patients with AIDS. Microbiol. 1989;12(1):101–4.
Bai QX, et al. Successful treatment of liver aspergilloma by caspofungin acetate first-line therapy in a non-immunocompromised patient. Int J Mol Sci. 2012;13(9):11063–70.
Potenza L, et al. Assessment of aspergillus-specific T cells for diagnosis of invasive aspergillosis in a leukemic child with liver lesions mimicking hepatosplenic candidiasis. Clin Vaccine Immunol. 2008;15(10):1625–8.
Marotta G, et al. Complete resolution of hepatic aspergillosis after non-myeloablative hematopoietic stem cell transplantation in a patient with acute myeloid leukemia. Hematol. 2005;10(5):383–6.
Gottfredsson M, Steingrímsdóttir H. Disseminated invasive aspergillosis in a patient with acute leukaemia. Acta Biomed. 2006;77(Suppl 2):10–3.
Bassetti M, et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin Infect Dis. 2021;72(Suppl 2):S121–s127.
Jha AK, et al. Evaluation of factors associated with complications in amoebic liver abscess in a predominantly toddy-drinking population: a retrospective study of 198 cases. JGH Open. 2019;3(6):474–9.
Neill L, et al. Clinical characteristics and treatment outcomes in a cohort of patients with pyogenic and amoebic liver abscess. BMC Infect Dis. 2019;19(1):490.
Wang YP, et al. Proton pump inhibitor use significantly increases the risk of cryptogenic liver abscess: a population-based study. Aliment Pharmacol Ther. 2015;41(11):1175–81.
Chen C-Y, et al. Chronic disseminated candidiasis manifesting as hepatosplenic abscesses among patients with hematological malignancies. BMC Infect Dis. 2019;19(1):635.
Chen L, et al. Adrenal and hepatic aspergillosis in an immunocompetent patient. Infect Dis (Lond). 2015;47(6):428–32.
Fiore M, et al. Liver fungal infections: an overview of the etiology and epidemiology in patients affected or not affected by oncohematologic malignancies. Infect Drug Resist. 2018;11:177–86.
Montrucchio G, et al. Risk factors for invasive aspergillosis in ICU patients with COVID-19: current insights and new key elements. Ann Intensive Care. 2021;11(1):136.
Waldeck F, et al. Influenza-associated aspergillosis in critically-ill patients-a retrospective bicentric cohort study. Eur J Clin Microbiol Infect Dis. 2020;39(10):1915–23.
Mohamed A, Rogers TR, Talento AF. COVID-19 associated invasive pulmonary aspergillosis: diagnostic and therapeutic challenges. J Fungi. 2020;6(3):115.
Stemler J, et al. Diagnosis and treatment of invasive aspergillosis caused by non-fumigatus aspergillus spp. J Fungi. 2023;9(4):500.
Kazan E, et al. A retrospective series of gut aspergillosis in haematology patients. Clin Microbiol Infect. 2011;17(4):588–94.
Singh A, et al. Prevalence of cases of amebic liver abscess in a tertiary care Centre in India: a study on risk factors, associated microflora and strain variation of Entamoeba histolytica. PLoS One. 2019;14(4):e0214880.
McCreery RJ, Florescu DF, Kalil AC. Sepsis in immunocompromised patients without human immunodeficiency virus. J Infect Dis. 2020;222(Supplement_2):S156–65.
Ergene U, et al. Disseminated aspergillosis due to aspergillus Niger in immunocompetent patient: a case report. Case Rep Infect Dis. 2013;2013:385190.
Ullmann AJ, et al. Diagnosis and management of aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–e38.
van Prehn J, et al. Hepatosplenic candidiasis without prior documented Candidemia: an Underrecognized diagnosis? Oncol. 2017;22(8):989–94.
Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–56.
Priyadarshi RN, Kumar R, Anand U. Amebic liver abscess: Clinico-radiological findings and interventional management. World J Radiol. 2022;14(8):272–85.
Jindal A, et al. Management practices and predictors of outcome of liver abscess in adults: a series of 1630 patients from a liver unit. J Clin Exp Hepatol. 2021;11(3):312–20.
Marr KA, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162(2):81–9.
Funding
Internal funding from Mayo Clinic.
Author information
Authors and Affiliations
Contributions
Conceptualization: ID, TM, MK, FS. Data collection: ID, ECM. Data analysis: ID, SMD, MR. Writing ID, IP, LRP, MA, AS, FS. Editing CN, FS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dumic, I., Caetano, E.M., Domingues, S.M. et al. Clinical characteristics, diagnosis, treatment, and outcome of patients with liver abscess due to Aspergillus spp: a systematic review of published cases. BMC Infect Dis 24, 345 (2024). https://doi.org/10.1186/s12879-024-09226-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09226-y