Abstract
Background
Anopheles stephensi is native to Southeast Asia and the Arabian Peninsula and has emerged as an effective and invasive malaria vector. Since invasion was reported in Djibouti in 2012, the global invasion range of An. stephensi has been expanding, and its high adaptability to the environment and the ongoing development of drug resistance have created new challenges for malaria control. Climate change is an important factor affecting the distribution and transfer of species, and understanding the distribution of An. stephensi is an important part of malaria control measures, including vector control.
Methods
In this study, we collected existing distribution data for An. stephensi, and based on the SSP1-2.6 future climate data, we used the Biomod2 package in R Studio through the use of multiple different model methods such as maximum entropy models (MAXENT) and random forest (RF) in this study to map the predicted global An. stephensi climatically suitable areas.
Results
According to the predictions of this study, some areas where there are no current records of An. stephensi, showed significant areas of climatically suitable for An. stephensi. In addition, the global climatically suitability areas for An. stephensi are expanding with global climate change, with some areas changing from unsuitable to suitable, suggesting a greater risk of invasion of An. stephensi in these areas, with the attendant possibility of a resurgence of malaria, as has been the case in Djibouti.
Conclusions
This study provides evidence for the possible invasion and expansion of An. stephensi and serves as a reference for the optimization of targeted monitoring and control strategies for this malaria vector in potential invasion risk areas.
Similar content being viewed by others
Introduction
Anopheles stephensi is a vector of Plasmodium vivax and Plasmodium falciparum parasites originating in Southeast Asia and the Arabian Peninsula [1,2,3]. Countries such as India, Afghanistan, Iran, Pakistan, Egypt, Myanmar, Thailand and China are the distribution areas of An. stephensi [4]. An. stephensi has been implicated in malaria transmission throughout much of its native range in Asia and the Middle East, including India, Iran, and Pakistan [5]. In India, An. stephensi is considered an efficient vector of urban malaria [3]. In China, another country with known distribution of An. stephensi, the mosquito is not a vector of malaria and has been reported to be distributed mainly in provinces of the oriental realm, such as Guangxi, Hainan, and Sichuan Provinces [6]. However, in recent years, there have been few reports of An. stephensi being collected in China [7]. Malaria is a life-threatening disease caused by parasites that are transmitted to humans through the bite of infected female Anopheles mosquitoes [8]. There are five parasite species that cause malaria in humans, and two of these species –P. vivax and P. falciparum– pose the greatest threat [8, 9]. According to the latest World Malaria Report, there were approximately 247 million malaria cases globally in 2021 (245 million in 2020), with an estimated 619,000 malaria deaths, and nearly half of the world's population is at risk of malaria [9]. Moreover, the number of malaria cases reported in Comoros, Costa Rica, Ecuador, Guatemala and some other countries on the mainland shows an increase compared to 2020 [9]. Malaria is considered one of the major vector-borne diseases most sensitive to changes in environmental conditions, similar to other vector-borne diseases such as dengue fever [10, 11]. This is because malaria incidence in endemic areas is largely determined by seasonal variations in mosquito populations and densities [12]. Additionally, environmental factors such as temperature [13] and precipitation [14, 15] can influence malaria incidence by altering the duration of the mosquito and parasite life cycles or by influencing human, vector or parasite behavior [16].
An. stephensi is a day-biting, anthropophilic mosquito found mainly in cattle sheds near human dwellings (endophilic) and which feeds indoors (endophagic) [3]. Research on An. stephensi in China [6] suggests that it breeds mainly in stagnant water in containers and can also breed in puddles, wells, and pools. Similar to Aedes mosquitoes, An. stephensi is able to breed in small, artificial containers in urban areas such as domestic water storage containers and garden reservoirs, and it can also breed and develop in larger water-containing structures [17,18,19] and seems to adapt quickly to local environments (including secluded habitats such as deep wells) [9]. During the dry season, when malaria transmission rates usually reach seasonal lows, An. stephensi can withstand extremely high temperatures [9], and this ability of the mosquito to adapt to different environments provides more possibilities for invasion and makes it more challenging to control.
An. stephensi is an invasive disease vector, and the evidence is increasing that the geographic range of An. stephensi has expanded over the last decade. Invasion by An. stephensi was first reported in Djibouti in 2012 [20], Notably, the invasion of An. stephensi in Djibouti in 2012 led to a 30-fold increase in malaria cases in Djibouti, from 1,684 cases in 2012 to 49,402 cases in 2019 [9, 19], and a 39-fold increase in malaria cases overall by 2020 [21]. A geostatistical model predicted that the species could spread to many other African cities, which would eventually expose at least 126 million people to risk [22]. Malaria is a major public health problem and challenge worldwide, and as an important vector of malaria, the adaptability of An. stephensi to urban environments compared to the main malaria vectors in Africa (An. gambiae and An. funestus) makes malaria control a greater challenge in more areas [23]. The invasion and population establishment of vector Anopheles mosquitoes in new areas brings possible opportunities for malaria transmission, so the invasion of these mosquitoes is also an important threat to global public health.
The shared socioeconomic pathways (SSPs) are new 'pathways' established by a series of international teams of climate scientists, economists and energy system modelers to study possible changes in global society, population and economy over the next century (https://www.carbonbrief.org/explainer-how-shared-socioeconomic-pathways-explore-future-climate-change/. Assessed Sep. 30, 2023). The SSP model is included in the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC), published in 2021, and has also been used to explore how societal choices will affect greenhouse gas emissions. The SSPs encompass five narratives of the future (SSP1-SSP5), and the SSP1 model describes a world of sustainability-focused growth and equality. By 2100, global carbon dioxide emissions will fall to approximately 22 to 48 gigatons per year (GtCO2), and the global surface temperature will rise by 3 to 3.5 °C under the SSP1 climate model (https://www.ipcc.ch/report/ar6/wg1/chapter/chapter-4/. Assessed Sep. 30, 2023). Climate change is an important factor influencing the distribution and transfer of species [24]. Climate change and increased carbon emissions may lead to rapid changes in the global distribution of vector mosquitoes [25], and these changes may even trigger the rapid spread of malaria into more regions.
Vector control is a very effective way to reduce malaria transmission and is an important component of malaria control and elimination strategies. The current research on the global distribution of An. stephensi is lacking, and systematic and large-scale surveillance of this vector is still in its infancy [9]. As invasion by An. stephensi is intensifying, understanding the current and future global potential areas suitable for the transmission of invasive mosquitoes is important for malaria control and prevention. Species distribution models (SDMs) are valuable and powerful tools for studying the effects of climate change on the potential distribution of species [26,27,28]. However, uncertainty of SDMs is prevalent due to differences in the use of ecological theories, as well as the assumptions of different SDMs and the different statistical methods used. An individual model may result in differences in suitable habitat for the same species due to multiple factors, leading to uncertainty in predictions [27, 28]. Biomod2 is a new computing framework developed in R for building SDMs [29], exploring the relationship between the spatial distribution of species and environmental variables through the use of multiple different model methods such as maximum entropy models (MAXENT) and random forest (RF), as well as calibrating and evaluating the models, thus improving the accuracy of predicting the potential distribution of species. Therefore, we used the Biomod2 package in R Studio in this study to map the predicted global An. stephensi climatically suitable areas, which can provide a necessary tool for assessing the current and future risk of An. stephensi.
Materials and Methods
Species occurrence data
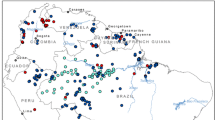
According to the species occurrence records from the literature (Table S1) and the Global Biodiversity Information Network website (https://www.gbif.org/, GBIF.org, 2019), we selected only those records that clearly originated from human observations, and from these, we screened records with precise latitude and longitude information to ensure geographic accuracy. Ultimately, a total of 964 global occurrence records for An. stephensi were obtained for further model construction (Fig. 1, Table S2).
Current bioclimatic variables
Current bioclimatic variables and elevation data with a spatial resolution of 2.5 m were both downloaded from WorldClim (https://www.Worldclim.org/. Assessed Sep. 16, 2023). The bioclimatic variables contain 19 climate factors (Bio1-Bio19, Table 1), and these variables were divided into 3 categories: (1) 9 temperature-related variables, Bio1, Bio2, Bio3, Bio4, Bio5, Bio6, Bio7, Bioo10 and Bio11. (2) 8 precipitation-related variables, Bio12, Bio13, bio14, Bio15, Bio16, Bio17, Bio18 and Bio19. (3) 2 coupling variables of temperature and precipitation, Bio8 and Bio9. These climate factors are closely related to the distribution of species and are necessary for prediction.
Future bioclimatic variables
The future bioclimatic variables were also obtained from worldclim (https://www.worldclim.org/). And the bioclimatic variables were under the new set of emissions scenarios Shared Socioeconomic Pathway (SSP) 1–2.6 (https://www.carbonbrief.org/explainer-how-shared-socioeconomic-pathways-explore-future-climate-change/#. Assessed Sep. 16, 2023). A number of these SSP scenarios have been selected to drive climate models for the Coupled Model Intercomparison Project 6 (CMIP6). The data of 2021–2040 and 2041–2060 were selected with the same spatial resolution of 2.5 m in this study.
Construction and evaluation of model
Ten different model were used in Biomod2, including artificial neural network (ANN) [30], classification and regression tree analysis (CTA) [31], flexible discriminant analysis (FDA) [32], generalized additive model (GAM) [33], generalized boosting model (GBM) [34], generalized linear models (GLM), multiple adaptive regression splines (MARS) [35], maximum entropy models (MAXENT) [36], random forest (RF) [37]and surface range envelope (SRE) [38]. 80% of the distribution records of An. stephensi were randomly selected as the training dataset and the remaining 20% as the testing dataset. Kappa coefficients (Kappa) [39], the true skill statistic (TSS) [40] and area under the receiver operating characteristic (ROC) curve (AUC) [41, 42]are used to evaluate the model. Generally, the higher the values of these indicators, the higher the accuracy of the model results [43].
Construction of ensemble model
The TSS values greater than or equal to 0.8 were selected from all individual models, and two integration methods, committee averaging (CA) and weighted mean of probabilities (WM), were used to integrate these models to produce an ensemble model (EM). Evaluation of the ensemble model produced by the two methods was carried out to select a method that performed better in the model evaluation index. The selected ensemble model was used to predict the potential distribution of An. stephensi under future climatic conditions for the years 2021–2040 and 2041–2060, and ultimately to derive a change in the distribution of An. stephensi by comparing it with the current climatically suitable areas.
Results
Model evaluation
The 10 individual models in Biomod2 were evaluated according to Kappa, TSS and AUC (Table 2). In terms of AUC values, RF has the highest AUC value in all the individual model, followed by GAM and MAXENT. In terms of TSS values, RF, GAM and MAXENT perform better. In terms of Kappa, CTA and SRE results are less than ideal. The best performing models include GAM, MAXENT, and RF models, which have higher values of Kappa, TSS, and AUC when compared to the other models. After selecting models with TSS values higher than 0.8 (GAM, GBM, MAXENT, RF) to construct the ensemble model, the Kappa, TSS and AUC values of the model were improved compared with those of the individual model. In addition, the ensemble model constructed through the WM method had higher Kappa and AUC when the AUC values were almost the same.
Current climatically suitable area prediction for An. stephensi
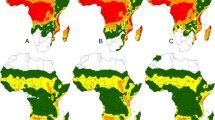
Figure 2 shows the suitability index distribution of climatically suitable areas for An. stephensi under the current climatic conditions based on the 10 individual model, and all the data have been normalized. It can be seen from the results that most of the climatically suitable areas of An. stephensi obtained from different single models are basically the same, although there are some differences in the distribution areas of An. stephensi. Totally, the global distribution of climatically suitable areas for An. stephensi is concentrated in parts of Asia and Africa between the Tropic of Cancer (Tropic of Cancer) and the Tropic of Capricorn (Tropic of Cancer), Southern Africa, parts of America and northern Australia.
The ensemble model (Fig. 3) constructed through the CA and WM methods predicted a similar range of climatically suitable areas for An. stephensi. Globally, areas of suitability for An. stephensi were concentrated in southern China, India, Pakistan, Afghanistan, Iran and most of Saudi Arabia, Africa, South America and a small portion of the southern United States, and some part of Australia. Overall, areas with a high suitability index for An. stephensi were found in most countries in South-East Asia, Saudi Arabia and Africa, particularly in most of the northern part of India bordering Myanmar, Myanmar, Vietnam, the southern coastal areas of China, Saudi Arabia and Algeria.
As can be seen from the model evaluation index (Table 2), the ensemble model constructed through the WM method has a higher Kappa and TSS than the ensemble model constructed through the CA method with the same AUC value, so we chose to use the ensemble model constructed through the WM method when making predictions of the global climatically suitable area of An. stephensi in the future climate scenarios.
Trends of the distribution of climatically suitable areas for An. stephensi under SSP1-2.6
Figure 4-A and B show the global climatically suitable areas for An. stephensi in 2021–2040 and 2041–2060, respectively, as predicted by the ensemble model constructed through the WM method based on the SSP1-2.6 climate scenarios. Comparing the results of the ensemble model prediction to the current prediction of climatically suitable areas, the global climatically suitable area for An. stephensi will expand by 33.17% from 2021–2040 (Fig. 5-A). From 2041–2060 (Fig. 5-B), the global climatically suitable area for An. stephensi will expand by 49.46%. We calculated the percentage of current and future climatically suitable areas for An. stephensi predicted by the ensemble model constructed through the WM method, and Table 3 provides a more intuitive view of future trends in the expansion of climatically suitable areas.
According to our predictions, the global climatically suitable areas of An. stephensi is expected to expand and move north-westwards over the next 40 years. The climatically suitable areas of Asia, Africa, America, Australia and other areas for An. stephensi are predicted to expand.
Discussion
The spread of An. stephensi is a major potential threat to malaria control and elimination in Africa and southern Asia. The propensity of An. stephensi to spread and establish outside the current range is already being observed, drawing the attention of the global health community (https://www.who.int/news/item/29-09-2022-who-launches-new-initiative-to-stop-the-spread-of-invasive-malaria-vector-in-africa. Assessed Oct. 13, 2023). Therefore, it is necessary to better understand the current distribution of An. stephensi and the potential future changes in its distribution. Individual SDMs have been used more frequently to predict the potential geographical distributions of invasive mosquitoes [44, 45], while the prediction results using individual SDMs may be under- or over-fitted [43, 46], and ensemble model is well suited to avoid or reduce this uncertainty. Therefore, in this study, we used global distribution data to model the global distribution of An. stephensi under current and future climate conditions using Biomod2, provided a map of the potential global distribution of An. stephensi under current climate conditions, and predicted changes in the global distribution of An. stephensi under future climate change conditions based on the SSP1-2.6 climate scenario. A total of 10 individual models were used in the construction of species distribution models using Biomod2. Among these models, MAXENT is the most commonly used model for species distribution prediction research using individual models [47]. By comparing the prediction results of MAXENT models and ensemble models in this study, we found that in terms of model evaluation indicators, when using the same species distribution data and climatic variables, the ensemble models have higher prediction accuracy. The prediction ranges of MAXENT and ensemble models are essentially the same, though, in terms of predicted climatically suitability areas maps. In addition, according to the prediction results of this study, the best performing models are GAM, MAXENT and RF, which have higher kappa, TSS and AUC values than the other models. These models also have higher predictive accuracy, leading to more accurate simulation effects. The differences in simulation effects between different models may be due to differences in how each model describes the fundamental and realised ecological niches of the species [48]. The ensemble model prediction method constructed in our study is a solution to reduce uncertainty, although it cannot solve the limitations and over-prediction of these individual models themselves, the results of this study show that the ensemble model prediction method is able to improve the model accuracy and reduce the model uncertainty to a certain extent.
Since An. stephensi was first reported in Djibouti in 2012, it was collected in Ethiopia and Sudan in 2016 [49, 50], Sri Lanka in 2017 [51], Somalia in 2019 [52], Nigeria in 2020 [53, 54] and Yemen in 2021 [21]. As recently as 2022, An. stephensi has also been collected in Kenya [21]. According to the predictions of this study, some areas where there are no current records of An. stephensi, especially in North Africa, showed significant areas of suitable habitat for An. stephensi. Ahn et al. [54] used bilateral maritime trade data to model and analyze countries with the highest risk of invasion of An. stephensi in Africa. The results showed that Djibouti and Sudan are the countries with the highest risk of invasion of An. stephensi in Africa, and An. stephensi has already invaded and established populations in these two countries. Strong maritime trade links seem to play a positive role in the invasion of An. stephensi and help it to invade new areas through human commerce. Countries such as Sudan, Egypt and Nigeria, as the known colonisation location for An. stephensi, are the bordering country to Chad, Libya, Niger and Central Africa, which are climatically suitable areas in our predicted results. Trade exchanges between regions are also an important influence on the invasive spread of species. 12 years ago, it might have been unknown to Africa, but 12 years later, An. stephensi has nearly invaded and colonised about a third of the African continent. It is justified to assume that these mosquitoes arrived on the African continent as a result of inter-country traffic and trade, accompanied by climate change, which facilitated their settlement. We consider, and our predictions suggest, that An. stephensi will spread in the future if no measures are taken.
Many studies have already demonstrated that An. stephensi comprises of three ecological variants, namely ‘type’ form, ‘intermediate’ and ‘mysorensis’ which can be characterized by egg morphometry [55,56,57]. The ‘type’ form is an efficient urban malaria vector in India due to its anthropophilic nature and adaptation to man-made breeding sites, moreover, ‘type’ and ‘intermediate’ forms have also emerged as efficient vectors in rural areas of India as a result of changing agricultural and water storage practices (https://www.who.int/publications/m/item/WHO-CDS-GMP-MPAC-2019.14. Assessed Dec. 13, 2023). But as far as the current research is concerned, we are unable to clearly distinguish these three variants [58]. There is currently no widely accepted molecular identification method for An. stephensi [59, 60]. Therefore, there is currently no precise documentation of the occurrence records of An. stephensi, as well as its new invasion records in Africa, which can accurately identify the ecological variants of An. stephensi. That is, most records of the distribution of An. stephensi are now sensu lato, not sensu stricto. We believe that further research is needed to develop reliable and feasible identification methods for these three variants of An. stephensi, and to accumulate distribution data for each genotype, in order to provide support for further distribution prediction and vector determination.
An. stephensi has been shown to be an effective vector of malaria in both rural and urban areas [61] and has a strong ability to survive and breed in urban areas [2], mainly in water tanks, water storage containers, construction sites, desert coolers, wells, and other artificial habitats [21, 61, 62], potentially placing urban populations at greater risk. In India, An. stephensi is the main malaria vector in urban environments and successfully sustains malaria transmission even at low vector densities [63, 64]. Sub-Saharan Africa, the region with the highest malaria burden, with more than 40% of the population living in urban environments [9], has many moderately and lowly suitable areas for An. stephensi in our projections, reinforcing the need to strengthen vector surveillance and control in these areas. In addition, the predictions showed that the southern United States, Mexico, and South America have large areas of moderately and lowly suitable habitat for An. stephensi. According to the latest WHO Malaria Report [9], Mexico and South America have the highest burden of malaria outside Sub-Saharan Africa, and in the United States, approximately 2,000 malaria cases are diagnosed annually, with the majority of these cases being imported [8]. Thus, once An. stephensi has successfully invaded, the suitable environment for its survival will help it to settle and spread in these areas, which will pose a new threat to malaria control. The sensitivity of malaria endemic vectors to insecticides is an important component of developing an effective vector management program [65]. Resistance in An. stephensi has been reported in Africa and Asia over the last two years. High resistance to pyrethroids was observed in An. stephensi mosquitoes captured in Ethiopia [66], which suggests the limited effectiveness of pyrethroid-only insecticide-treated nets (which have been used throughout Ethiopia) in controlling malaria transmitted by An. stephensi. Resistance in An. stephensi has also been reported in regions such as Afghanistan [67], Pakistan [68], Dubai [69] and India [70]. The development of resistance in malaria vectors is one of the serious limitations to effective vector control strategies that rely on chemical insecticides [65], and the resistance shown by An. stephensi to insecticides in these areas poses an additional challenge to its control. Therefore, to cope with the risk of invasion and malaria transmission associated with the expansion of An. stephensi habitats, integrated vector control measures should be actively strengthened in these areas. In terms of protecting populations, attention should be given to improving health education, housing conditions and the use of screens and other barriers to prevent invasive mosquitoes from entering human dwellings, and consideration should be given to adopting or intensifying the use of insecticide-treated mosquito nets or indoor residual spraying. Therefore, in suitable areas where An. stephensi has not yet invaded, mosquito species should be routinely monitored, identified and documented to prevent the importation of An. stephensi, especially in locations where there is movement of people and trade, such as airports, seaports, land ports and other ports of exit and entry.
The invasive spread of malaria vectors has affected the control programs of many malaria-endemic countries in Africa and Asia [9, 71, 72], posing a great challenge to the control of malaria and its vectors. The predicted results of this study, however, provide evidence for the possible invasion and expansion of An. stephensi as well as a reference base for the optimization of targeted surveillance and control strategies for An. stephensi in potential invasion risk areas.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Ahmed A, Khogali R, Elnour MB, Nakao R, Salim B. Emergence of the invasive malaria vector Anopheles stephensi in Khartoum State, Central Sudan. Parasit Vectors. 2021;14(1):511.
Tadesse FG, Ashine T, Teka H, Esayas E, Messenger LA, Chali W, Meerstein-Kessel L, Walker T, Wolde Behaksra S, Lanke K, et al. Anopheles stephensi Mosquitoes as Vectors of Plasmodium vivax and falciparum, Horn of Africa, 2019. Emerg Infect Dis. 2021;27(2):603–7.
Ishtiaq F, Swain S, Kumar SS. Anopheles stephensi (Asian Malaria Mosquito). Trends Parasitol. 2021;37(6):571–2.
Dong XS, Zhou HN. A catalog of the mosquito of the Yunnan, vol. 5. Kunming: Yunnan Science and Technology Press; 2019.
Ryan SJ, Lippi CA, Villena OC, Singh A, Murdock CC, Johnson LR. Mapping current and future thermal limits to suitability for malaria transmission by the invasive mosquito Anopheles stephensi. Malar J. 2023;22(1):104.
Lu BL: Fauna sinica:Insecta.Diptera: Culicidae 2, vol. 9. Beijing: China Science Press 1997.
Yan ZT, Yang FL. FU WB, Li XD, Yu G, Chen B: A Revised Checklist of Anopheles Species in China (Diptera: Culicidae). Journal of Chongqing Normal University. 2013;30(6):36–45.
Walter K, John CC. Malaria Jama. 2022;327(6):597.
WHO: World malaria report 2022. In. Geneva; 2022.
WHO: World malaria report 2011. In. Geneva; 2011.
Parham PE, Michael E. Modeling the effects of weather and climate change on malaria transmission. Environ Health Perspect. 2010;118(5):620–6.
Beloconi A, Nyawanda BO, Bigogo G, Khagayi S, Obor D, Danquah I, Kariuki S, Munga S, Vounatsou P. Malaria, climate variability, and interventions: modelling transmission dynamics. Sci Rep. 2023;13(1):7367.
Rodó X, Martinez PP, Siraj A, Pascual M. Malaria trends in Ethiopian highlands track the 2000 ‘slowdown’ in global warming. Nat Commun. 2021;12(1):1555.
Roy M, Bouma MJ, Ionides EL, Dhiman RC, Pascual M. The potential elimination of Plasmodium vivax malaria by relapse treatment: insights from a transmission model and surveillance data from NW India. PLoS Negl Trop Dis. 2013;7(1): e1979.
Laneri K, Bhadra A, Ionides EL, Bouma M, Dhiman RC, Yadav RS, Pascual M. Forcing versus feedback: epidemic malaria and monsoon rains in northwest India. PLoS Comput Biol. 2010;6(9): e1000898.
Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, Patz JA: Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environ Health Perspect 2001, 109 Suppl 2(Suppl 2):223–233.
Takken W, Lindsay S. Increased Threat of Urban Malaria from Anopheles stephensi Mosquitoes. Africa Emerg Infect Dis. 2019;25(7):1431–3.
Ganguly KS, Modak S, Chattopadhyay AK, Ganguly KS, Mukherjee TK, Dutta A, Biswas D: Forecasting Based On a SARIMA Model of Urban Malaria for Kolkata. Computer Science Department, Birla Institute of Technology, Mesra, Ranchi, Jharkhand 835215, India;Department of Statistics, University of Calcutta, Kolkata, West Bengal 700019, India;Health Depart 2016, Vol.4(No.2):22–33.
Doumbe-Belisse P, Kopya E, Ngadjeu CS, Sonhafouo-Chiana N, Talipouo A, Djamouko-Djonkam L, Awono-Ambene HP, Wondji CS, Njiokou F, Antonio-Nkondjio C. Urban malaria in sub-Saharan Africa: dynamic of the vectorial system and the entomological inoculation rate. Malar J. 2021;20(1):364.
Faulde MK, Rueda LM, Khaireh BA. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti. Horn of Africa Acta Trop. 2014;139:39–43.
Al-Eryani SM, Irish SR, Carter TE, Lenhart A, Aljasari A, Montoya LF, Awash AA, Mohammed E, Ali S, Esmail MA, et al. Public health impact of the spread of Anopheles stephensi in the WHO Eastern Mediterranean Region countries in Horn of Africa and Yemen: need for integrated vector surveillance and control. Malar J. 2023;22(1):187.
Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, Willis KJ. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci U S A. 2020;117(40):24900–8.
Samarasekera U. A missed opportunity? Anopheles stephensi in Africa Lancet. 2022;400(10367):1914–5.
Hickling R, Roy DB, Hill JK. Thomas CDJGCB: A northward shift of range margins in British Odonata. 2010;11(3):502–6.
Kamal M, Kenawy MA, Rady MH, Khaled AS, Samy AM. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae albopictus under changing climate. PLoS One. 2018;13(12):0210122.
McQuillan MA, Rice AM. Differential effects of climate and species interactions on range limits at a hybrid zone: potential direct and indirect impacts of climate change. Ecol Evol. 2015;5(21):5120–37.
Guo Y, Li X, Zhao Z, Nawaz Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. The Science of the total environment. 2019;648:1–11.
Zheng J, Wei H, Chen R, Liu J, Wang L, Gu W: Invasive Trends of Spartina alterniflora in the Southeastern Coast of China and Potential Distributional Impacts on Mangrove Forests. Plants (Basel, Switzerland) 2023, 12(10).
Anderson RP. A framework for using niche models to estimate impacts of climate change on species distributions. Ann N Y Acad Sci. 2013;1297:8–28.
Hirzel AH, Hausser J, Chessel D, Perrin N. Ecological-niche factor analysis: how to compute habitat-suitability maps without absence data? Ecology. 2002;83(7):2027–36.
Loh W-Y. Classification and regression trees. WIREs Data Min Knowl Discovery. 2011;1(1):14–23.
Hastie T, Tibshirani R, Buja A: Flexible Discriminant Analysis by Optimal Scoring. Journal of the American Statistical Association 2000, 89.
Leathwick JR, Elith J, Hastie T. Comparative performance of generalized additive models and multivariate adaptive regression splines for statistical modelling of species distributions. Ecol Model. 2006;199(2):188–96.
Ridgeway G: Generalized boosted models: A guide to the gbm package. 2005.
Jerome HF. Multivariate Adaptive Regression Splines. Ann Stat. 1991;19(1):1–67.
Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190(3):231–59.
Lopatin J, Dolos K, Hernández HJ, Galleguillos M, Fassnacht FE. Comparing Generalized Linear Models and random forest to model vascular plant species richness using LiDAR data in a natural forest in central Chile. Remote Sens Environ. 2016;173:200–10.
Busby JR. BIOCLIM - a bioclimate analysis and prediction system. Plant Prot Q. 1991;6:8–9.
De Raadt A, Warrens MJ, Bosker RJ, Kiers HAL. Kappa Coefficients for Missing Data. Educ Psychol Measur. 2019;79(3):558–76.
ALLOUCHE O, TSOAR A, KADMON R: Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). 2006, 43(6):1223–1232.
Bell JF, Fielding AH. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24(1):38–49.
Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–43.
Xian X, Zhao H, Wang R, Huang H, Chen B, Zhang G, Liu W, Wan F. Climate change has increased the global threats posed by three ragweeds (Ambrosia L) in the Anthropocene. The Science of the total environment. 2023;859(pt2):160252.
Liu Q, Zhang HD, Xing D, Jia N, Du YT, Xie JW, Wang M, Li CX, Zhao T, Jiang YT et al: The predicted potential distribution of Aedes albopictus in China under the shared socioeconomic pathway (SSP)1–2.6. Acta Trop 2023, 248:107001.
Liu Q, Xie JW, Wang M, Du YT, Yin ZG, Zhou NX, Zhao TY, Huang EJ, Zhang HD: Potential Global Distribution of the Invasive Mosquito Aedes koreicus under a Changing Climate. Tropical medicine and infectious disease 2023, 8(10).
Townsend Peterson A, Papeş M, Eaton M. Transferability and model evaluation in ecological niche modeling: a comparison of GARP and Maxent. Ecography. 2007;30(4):550–60.
Ahmed SE, McInerny G, O’Hara K, Harper R, Salido L, Emmott S, Joppa LN. Scientists and software – surveying the species distribution modelling community. Divers Distrib. 2015;21(3):258–67.
Bi Y-F, Xu J, Li Q-H, Guisan A, Thuiller W, Zimmermann NE, Yang Y, Yang X-F, Lausanne dLC, Alpine LdEJABY: Applying BioMod for Model-Ensemble in Species Distributions:a Case Study for Tsuga chinensis in China. 2013:647–655.
Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, Ibrahim M, Mohammed S, Janies D. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018;188:180–6.
Ahmed A, Pignatelli P, Elaagip A, Abdel Hamid MM, Alrahman OF, Weetman D. Invasive Malaria Vector Anopheles stephensi Mosquitoes in Sudan, 2016–2018. Emerg Infect Dis. 2021;27(11):2952–4.
Surendran SN, Sivabalakrishnan K, Gajapathy K, Arthiyan S, Jayadas TTP, Karvannan K, Raveendran S, Parakrama Karunaratne SHP, Ramasamy R. Genotype and biotype of invasive Anopheles stephensi in Mannar Island of Sri Lanka. Parasit Vectors. 2018;11(1):3.
Ali S, Samake JN, Spear J, Carter TE. Morphological identification and genetic characterization of Anopheles stephensi in Somaliland. Parasit Vectors. 2022;15(1):247.
WHO: WHO initiative to stop the spread of Anopheles stephensi in Africa. In. Edited by Organization WH; 2022.
Ahn J, Sinka M, Irish S, Zohdy S. Modeling marine cargo traffic to identify countries in Africa with greatest risk of invasion by Anopheles stephensi. Sci Rep. 2023;13(1):876.
Tyagi V, Dhiman S, Sharma A, Srivastava AR, Rabha B, Veer V. Morphometric and morphological appraisal of the eggs of Anopheles stephensi (Diptera: Culicidae) from India. J Vector Borne Dis. 2017;54:151–6.
Subbarao SK, Vasantha K, Adak T, Sharma VP, Curtis CF. Egg-float ridge number in Anopheles stephensi: ecological variation and genetic analysis. Med Vet Entomol. 1987;1(3):265–71.
Alam MT, Bora H, Das MK, Sharma YD. The type and mysorensis forms of the Anopheles stephensi (Diptera: Culicidae) in India exhibit identical ribosomal DNA ITS2 and domain-3 sequences. Parasitol Res. 2008;103(1):75–80.
Gakhar SK, Sharma R, Sharma A. Population genetic structure of malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Indian J Exp Biol. 2013;51(4):273–9.
Khan J, Gholizadeh S, Zhang D, Wang G, Guo Y, Zheng X, Wu Z, Wu Y. Identification of a biological form in the Anopheles stephensi laboratory colony using the odorant-binding protein 1 intron I sequence. PLoS ONE. 2022;17(2): e0263836.
Singh OP, Mishra S, Sharma G, Sindhania A, Kaur T, Sreehari U, Das MK, Kapoor N, Gupta B. Evaluation of intron-1 of odorant-binding protein-1 of Anopheles stephensi as a marker for the identification of biological forms or putative sibling species. PLoS ONE. 2022;17(7): e0270760.
Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, Gething PW, Elyazar IR, Kabaria CW, Harbach RE, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89.
Sharma RS. Urban malaria and its vectors Anopheles stephensi and Anopheles culicifacies (Diptera : Culicidae) in Gurgaon, India. The Southeast Asian journal of tropical medicine and public health. 1995;26(1):172–6.
Sharma SN, Subbarao SK, Choudhury DS, Pandey KC: Role of An. culicifacies and An. stephensi in malaria transmission in urban Delhi. Indian journal of malariology 1993, Vol.30(No.3):155–168.
Subbarao SK, Nanda N, Rahi M, Raghavendra K. Biology and bionomics of malaria vectors in India: existing information and what more needs to be known for strategizing elimination of malaria. Malar J. 2019;18(1):396.
Zare M, Soleimani-Ahmadi M, Davoodi SH, Sanei-Dehkordi A. Insecticide susceptibility of Anopheles stephensi to DDT and current insecticides in an elimination area in Iran. Parasit Vectors. 2016;9(1):571.
Balkew M, Mumba P, Yohannes G, Abiy E, Getachew D, Yared S, Worku A, Gebresilassie A, Tadesse FG, Gadisa E, et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar J. 2021;20(1):263.
Safi NH, Ahmadi AA, Nahzat S, Ziapour SP, Nikookar SH, Fazeli-Dinan M, Enayati A, Hemingway J. Evidence of metabolic mechanisms playing a role in multiple insecticides resistance in Anopheles stephensi populations from Afghanistan. Malar J. 2017;16(1):100.
Hemingway J. The biochemical nature of malathion resistance in Anopheles stephensi from Pakistan. Pesticide Biochemistry and Physiology. 1982;17(2):149–55.
Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J. Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 2003;17(2):138–44.
Hariprasad TP, Shetty NJ. Biochemical basis of alphamethrin resistance in different life stages of Anopheles stephensi strains of Bangalore. India Pest Manag Sci. 2016;72(9):1689–701.
Raghavendra K, Barik TK, Reddy BP, Sharma P, Dash AP. Malaria vector control: from past to future. Parasitol Res. 2011;108(4):757–79.
Vatandoost H, Hanafi-Bojd AA. Indication of pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pac J Trop Med. 2012;5(9):722–6.
Acknowledgements
Not applicable.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, Tong-Yan Zhao, Heng-Duan Zhang; methodology, Heng-Duan Zhang; software, Heng-Duan Zhang; validation, Qing Liu; formal analysis, Qing Liu; data curation, Ming Wang, Yu-Tong Du, Jing-Wen Xie, Zi-Ge Yin and Jing-Hong Cai; writing—original draft preparation, Qing Liu; writing—review and editing, Tong-Yan Zhao and Heng-Duan Zhang. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Q., Wang, M., Du, YT. et al. Possible potential spread of Anopheles stephensi, the Asian malaria vector. BMC Infect Dis 24, 333 (2024). https://doi.org/10.1186/s12879-024-09213-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09213-3