Abstract
Background
Currently, culture methods are commonly used in clinical tests to detect pathogenic fungi including Candida spp. Nonetheless, these methods are cumbersome and time-consuming, thereby leading to considerable difficulties in diagnosis of pathogenic fungal infections, especially in situations that respiratory samples such as alveolar lavage fluid and pleural fluid contain extremely small amounts of microorganisms. The aim of this study was to elucidate the utility and practicality of microfluidic chip technology in quick detection of respiratory pathogenic fungi.
Methods
DNAs of clinical samples (mainly derived from sputa, alveolar lavage fluid, and pleural fluid) from 64 coastal patients were quickly detected using microfluidic chip technology with 20 species of fungal spectrum and then validated by Real-time qPCR, and their clinical baseline data were analyzed.
Results
Microfluidic chip results showed that 36 cases infected with Candida spp. and 27 cases tested negative for fungi, which was consistent with Real-time qPCR validation. In contrast, only 16 cases of fungal infections were detected by the culture method; however, one of the culture-positive samples tested negative by microfluidic chip and qPCR validation. Moreover, we found that the patients with Candida infections had significantly higher rates of platelet count reduction than fungi-negative controls. When compared with the patients infected with C. albicans alone, the proportion of males in the patients co-infected with multiple Candidas significantly increased, while their platelet counts significantly decreased.
Conclusions
These findings suggest that constant temperature amplification-based microfluidic chip technology combined with routine blood tests can increase the detection speed and accuracy (including sensitivity and specificity) of identifying respiratory pathogenic fungi.
Similar content being viewed by others
Background
The growth and reproduction of microorganisms require appropriate temperature and humidity. In particular, fungi prefer a humid and warm environment, and the optimum temperature for fungal growth is around 30 °C. Fungi are present everywhere in the environment such as atmosphere, plants, animals, feces, floors, and soil [1]. During spring and summer in Southern China, high-temperature and humidity environments are especially suitable for the growth and reproduction of fungi, and thereby increase risk of pathogenic fungal infections in population with long-term exposure to warm and humid environments. Older individuals and patients with chronic respiratory diseases are susceptible to respiratory fungal colonization and invasion [2]. Generally, fungal infections in the respiratory tract are dominated by Candida spp., especially C. albicans [3].
Detection methods such as 1,3-beta-D-glucan assay (G test) [4], microscopic examination [5], culture methods [6], histopathology [7], serology [8], and molecular biology [9] have been used in diagnosis of fungal infections. Traditional culture methods are widely used to characterize pathogenic fungi including Candida spp. However, culture-based methods are cumbersome, time-consuming, and thus unsuitable for rapid detection. Even more, the results are generally false negative. G test is used to detect one of the main structural components of the fungal cell wall (1,3-beta-D-glucan), but cannot distinguish fungal species. Despite the accuracy and efficacy of PCR-based methods [10], using a pair of primers or probes can only detect one type of the fungi; even multiplex PCR can detect no more than 5 Candida spp [11]. Therefore, bio-chips including microfluidic chip are becoming an alternative and revolutionary technology for assaying multiple fungi.
In this study, we aimed to rapidly detect the pathogenic fungi in coastal population with respiratory infectious diseases using microfluidic technology. To verify the accuracy of microfluidic chip, the results were further validated using Real-time qPCR and the clinical baseline data of the patients with or without fungal infections were analyzed.
Methods
Respiratory samples of patients
Patients with severe respiratory infections and hospitalized in the Department of Critical Care Medicine and Department of Respiratory and Critical Care Medicine between July 2020 and June 2021 at the Affiliated Hospital of Guangdong Medical University (Zhanjiang, China) were selected in this study. The patients had to be aged ≥ 18 years and their respiratory samples (sputum, alveolar lavage fluid, pleural fluid, etc.) were collected and sent to the department of precision laboratory for inspection. Cases who refused to accept the chip-based detection of pathogenic fungi were excluded. This study was approved by the ethics committee of the Affiliated Hospital of Guangdong Medical University (approval No. KT2022-119-01) and the consent of all patients was obtained.
Clinical data collection
Clinical baseline data (including age, gender, fever, cough, sputum production, coma, use of antifungal agents, white blood cells, neutrophils, lymphocytes, red blood cells, platelets, glucose, total protein, albumin, and globulin) and microbiological culture results were obtained from electronic medical records (Table S1).
Microfluidic-based detection of pathogenic fungi
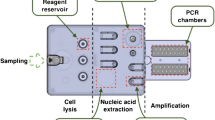
The genomic DNAs of respiratory samples were isolated using the nucleic acid extraction kit (CapitalBio Technology, China) and subsequently used for chip-based detection of pathogenic fungi. The butterfly-shaped microfluidic chip system for rapid detection of broad-spectrum fungi was customized from CapitalBio Technology and prepared by constant temperature amplification with microfluidic technologies. The reaction of microfluidic-based detection was carried out on a microfluidic disk chip. Each chip had 24 reaction pools, in which were embedded with a set of specific primers targeting 20 species of pathogenic fungi. The fluorescent dye incorporation method was used, and the isothermal amplification technology adopted was loop-mediated isothermal amplification (LAMP). Its principle is based on the fact that DNA is in a dynamic equilibrium state at about 65℃. When any primer carries out base pairing extension to the complementary position of double-stranded DNA, the other strand will dissociate and become a single strand. After the one-step amplification and detection of target sequences, the samples generating snake-shaped amplification curves represented positive signals.
Validation using real-time qPCR
According to the detection results of microfluidic chip, the specific primers of pathogenic Candida spp. including C. albicans, C. tropicalis, C. glabrata, C. parapsilosis, and C. krusei were synthesized for qPCR validation. Real-time qPCR was performed according to the specification of 2×RealStar Green Power Mixture with ROX (GenStar, China) by Applied Biosystems 7300 Real-Time PCR System (Thermo, USA). The cycling conditions were as follows: a pre-run at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 15 s, followed by a 60 °C annealing for 45 s and a 72 °C extension for 30 s; the melting curve conditions were automatically set up. The Real-time qPCR reactions were performed in triplicate and GAPDH was used as internal reference. Finally, the amplified products were electrophoresed on 1.0% agarose gels and developed using the nucleic acid dye Gelred (Biotium, USA). The sequences of PCR primers were listed in Table S2.
Statistical analysis
The measurement data that conformed to a normal distribution were expressed as mean ± standard deviation (SD), and the t-test was used for the comparison between the two groups; the measurement data that did not conform to the normal distribution were expressed as median and interquartile range (IQR), and the comparison between the two groups was conducted by Mann-Whitney U test. The count data were expressed as the number of cases and percentage, and the difference was analyzed by chi-square test or Fisher’s exact value test. P < 0.05 was considered to demonstrate statistically significant differences.
Results
Fungal species detected by culture method and microfluidic chip technology
A total of 64 patients with clinical infections were included in this study, and their detected results were finished by the microfluidic chip technologies (Fig. S1). Among the patients recruited, the mean age was 60.50 ± 16.44 years and 44 patients (68.75%) were male (Table S1). Only 8 cases with C. albicans, 1 case with C. glabrata, 2 cases with A. fumigatus, 1 case with A. flavus, 3 cases with small amounts of sporozoites, and 1 case with unidentified fungi were detected by culture method (Fig. 1A). In contrast, 36 cases with Candida spp. (including C. albicans, C. tropicalis, C. glabrata, C. parapsilosis, and C. krusei) and 1 case with A. fumigatus were detected using the microfluidic chip; and two of those cases infected with Candida spp. were co-infected with T. asahii and A. fumigatus, respectively (Fig. 1B). Confusingly, one of the C. albicans-positive samples detected by culture method was negative in the microfluidic chip. These findings imply that microfluidic-based chip system is a technique much more sensitive than traditional culture method.
qPCR-based validation for chip detection
Real-time qPCR was performed for validation, and the results were consistent with the chip detection results (Fig. 2). We found that the case with C. albicans positive in cultures but negative in the microfluidic chip showed no obvious amplification, and thus it was judged as negative. The qPCR validation indicated that microfluidic chip technology is more accurate in diagnosing fungal infections (Fig. S2).
The accuracy for detection of Candida spp.
The patients were divided into case group (Candida infection; n = 36) and control group (fungal-negative; n = 27) according to the standard qPCR assay. As shown in Table 1, the sensitivity and specificity of microfluidic chip technology were both 100%, while the sensitivity of culture method was 22.22%. This finding suggests that the accuracy of microfluidic chip technology was superior to that of culture method.
Clinical features of patients infected with Candida spp
The clinical baseline data were compared between the patients infected with Candida spp. (n = 36) and fungal-negative controls (n = 27) detected by microfluidic chip. As shown in Table 2, age, gender, fever, cough, sputum production, coma, use of antifungal agents, and laboratory examination indexes (including white blood cells, neutrophils, red blood cells, platelets, blood glucose, total protein, albumin, and globulin) were not significantly different between the two groups, except for the proportion of abnormal platelet counts (P = 0.038). The results suggest that Candida infections may affect platelets.
Clinical features of patients co-infected with C. albicans and other Candidas
As shown in Table 3, when compared with the patients infected with C. albicans alone (n = 18), the proportion of males in the patients co-infected with C. albicans and other Candidas (n = 10) significantly increased (P = 0.048), while the platelet counts significantly decreased (P = 0.021). These findings indicate that Candida complex infections which are more likely to occur in males than females may lead to severe thrombocytopenia.
Discussion
While microfluidic chip has been used for rapid detection of fungal infections [12], it is still a bottleneck problem to test special clinical samples via high-throughput assays. To detect low-density fungi in body fluid, we customized the microfluidic-based chip system which allows timely detection of broad-spectrum pathogenic fungi, including C. albicans, C. tropicalis, C. glabrata, C. parapsilosis, C. krusei, C. auris, A. fumigatus, A. flavus, A. niger, C. neoformans, T. asahii, etc. It is shown that the pathogenic fungi detected by culture method and chip technology mainly consisted of Candidas, but the detection rate of microfluidic chip technology were higher than that of culture method (Fig. 1). The molecular detection results of Candidas using the microfluidic chip were validated by Real-time qPCR and showed good agreement (Fig. 2). Moreover, the blood routine examination implies that the platelets counts may be reduced in the patients with Candida infections and even associated with infection severity (Tables 2 and 3).
Invasive fungal infections are the most common causes of high mortality in ICU, and prompt antifungal therapy is a critical determinant for good clinical outcomes [13]. However, the treatment of fungal infections is often delayed due to the insensitivity and slow steps of microbial culture. Therefore, the development and validation of non-culture diagnostics targeting pathogenic fungi are of utmost priority in medicine. While commercial PCR detection has been widely applied to fungal examination, the use of DNA chip is becoming the method of choice for profiling fungal gene expression [14, 15]. The results of microbial culture showed only fungal infections in 16 of 64 patients, in which one was negative by microfluidic chip and qPCR validation (Fig. 1 and 2; Table 1). In contrast, 36 cases that test Candida positive by microfluidic chip were consistent with the qPCR validation (Figs. 1 and 2; Table 1), suggesting that the sensitivity and accuracy of microfluidic chip technology for fungal detection are more superior than culture methods. Furthermore, the microfluidic chip can simultaneously detect 20 species of pathogenic fungi, suggesting that it has the advantage of high-throughput detection compared with conventional PCR methods.
Candida spp. especially C. albicans play essential roles in invasive fungal infections. The development of diagnosis and treatment methods increases the risk of Candida infections [16,17,18], for instance, the use of broad-spectrum antibiotics, immunosuppressants, total parenteral nutrition, and mechanical ventilation leads to microbial dysbiosis. According to common sense, pathogenic microorganism infections cause blood-routine changes. However, we found that white blood cell counts were not significantly different between the patients infected with Candida spp. and fungal-negative controls (Table 2), probably due to additional bacterial infections.
As multiple players in innate immunity, platelets interact with Candida spp. and may contribute to the fatal outcome of invasive Candida infections [19, 20]. Thrombocytopenia, an important risk factor for fungal infections was also observed in the patients infected with Candidas (Table 3). It is suggested that Candidas enhance platelet adherence [19]. Platelets cannot directly reflect Candida infections, but may favor the survival of invasive Candidas in blood [21]. We found that platelet counts decreased in some patients with Candida infections in respiratory tract (Table 3), which is consistent with previous literature reports [22, 23], suggesting that Candida spp. may also be present in their blood and hence cause platelet aggregation and destruction. The mechanism by which platelets adhere to Candidas depends on the species of Candidas [20]. A study showed that C. albicans was able to evade complement-mediated killing without platelet adherence, and thus survived in blood during the infection period [24]. Moreover, it is showed that thrombocytopenia in the patients co-infected with C. albicans and other Candidas was more severe than those infected with C. albicans alone (Table 3). These findings suggest that platelet counts may be used as a parameter to investigate the severity of invasive Candida infections. However, the number of the patients in each group is small. Therefore, future studies should expand the sample size to observe and explore the relationship between platelets and Candida infections.
Conclusions
In summary, microfluidic chip technology combined with routine blood tests can improve the accuracy and efficacy of detection of respiratory pathogenic Candida spp. However, the suitability of microfluidic chip technology for detecting clinically relevant fungal infections in other tissues and organs remains to be further explored.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Gadd G, Watkinson S, Dyer P, editors. Fungi in the environment (British Mycological Society Symposia). Cambridge: Cambridge University Press; 2007.
Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel). 2017;3(4):57.
Pendleton KM, Huffnagle GB, Dickson RP. The significance of Candida in the human respiratory tract: our evolving understanding. Pathog Dis. 2017;75(3):ftx029.
Tran T, Beal SG. Application of the 1,3-β-D-glucan (Fungitell) assay in the diagnosis of invasive fungal infections. Arch Pathol Lab Med. 2016;140(2):181–5.
Abe M, Kume H. Clinical mycological examination–direct microscopic examination and isolation of clinical specimens. Med Mycol J. 2013;54(1):19–25.
Babb J, Clark A, Gaffney D, Abdelfattah K, Prokesch BC. Little utility of fungal blood cultures in surgical and burn intensive care units. Microbiol Spectr. 2022;10(4):e0022822.
Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24(2):247–80.
Fréalle E, Valot S, Piarroux R, Menotti J, Lachaud L, Persat F, et al. Update on the diagnosis of parasitic and fungal infections. Ann Biol Clin (Paris). 2020;78(3):299–313.
Zhu A, Zembower T, Qi C. Molecular detection, not extended culture incubation, contributes to diagnosis of fungal infection. BMC Infect Dis. 2021;21(1):1159.
Mendonça A, Santos H, Franco-Duarte R, Sampaio P. Fungal infections diagnosis - past, present and future. Res Microbiol. 2022;173(3):103915.
Clancy CJ, Nguyen MH. Non-culture diagnostics for invasive candidiasis: promise and unintended consequences. J Fungi (Basel). 2018;4(1):27.
Asghar W, Sher M, Khan NS, Vyas JM, Demirci U. Microfluidic chip for detection of fungal infections. ACS Omega. 2019;4(4):7474–81.
Kochanek M, Köhler P. Invasive fungal infections in ICU patients - what’s New? Dtsch Med Wochenschr. 2021;146(7):455–60.
Lu W, Gu D, Chen X, Xiong R, Liu P, Yang N, et al. Application of an oligonucleotide microarray-based nano-amplification technique for the detection of fungal pathogens. Clin Chem Lab Med. 2010;48(10):1507–14.
Sturaro LL, Gonoi T, Busso-Lopes AF, Tararam CA, Levy CE, Lyra L, et al. Visible DNA microarray system as an adjunctive molecular test in identification of pathogenic fungi directly from a blood culture bottle. J Clin Microbiol. 2018;56(5):e01908–17.
Clancy CJ, Pappas PG, Vazquez J, Judson MA, Kontoyiannis DP, Thompson GR 3rd, et al. Detecting infections rapidly and easily for candidemia trial, part 2 (DIRECT2): a prospective, multicenter study of the T2Candida panel. Clin Infect Dis. 2018;66(11):1678–86.
Clancy CJ, Nguyen MH. Diagnostic methods for detection of blood-borne candidiasis. Methods Mol Biol. 2016;1356:215–38.
Knitsch W, Vincent JL, Utzolino S, François B, Dinya T, Dimopoulos G, et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis. 2015;61(11):1671–8.
Robert R, Nail S, Marot-Leblond A, Cottin J, Miegeville M, Quenouillere S, et al. Adherence of platelets to Candida species in vivo. Infect Immun. 2000;68(2):570–6.
Speth C, Rambach G, Lass-Flörl C. Platelet immunology in fungal infections. Thromb Haemost. 2014;112(4):632–9.
Eberl C, Speth C, Jacobsen ID, Hermann M, Hagleitner M, Deshmukh H, et al. Candida: platelet interaction and platelet activity in vitro. J Innate Immun. 2019;11(1):52–62.
Hammoud MS, Al-Taiar A, Fouad M, Raina A, Khan Z. Persistent candidemia in neonatal care units: risk factors and clinical significance. Int J Infect Dis. 2013;17(8):e624–8.
Nieto-Rodriguez JA, Kusne S, Mañez R, Irish W, Linden P, Magnone M, et al. Factors associated with the development of candidemia and candidemia-related death among liver transplant recipients. Ann Surg. 1996;223(1):70–6.
Willcox MD, Webb BC, Thakur A, Harty DW. Interactions between Candida species and platelets. J Med Microbiol. 1998;47(2):103–10.
Acknowledgements
Not applicable.
Funding
This work was supported by the Discipline Construction Project of Guangdong Medical University (4SG21233G), Southern Marine Science and Engineering Guangdong Laboratory Zhanjiang (ZJW-2019-007), Key platform of Department of Education of Guangdong Province (2021LSYS007), and Zhanjiang Science and Technology Development Special Funding Competitive Allocation Project (2022E05011, 2022A01196, 2021A05158).
Author information
Authors and Affiliations
Contributions
QY and DC performed the experiments and collected the data. LD and YZ provided the experimental materials. YH and QY organize and analyzed the data. YH wrote the manuscript. WL and HL designed this study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the Affiliated Hospital of Guangdong Medical university (approval No. KT2022-119-01). Written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yao, Q., He, Y., Deng, L. et al. Rapid detection of pathogenic fungi from coastal population with respiratory infections using microfluidic chip technology. BMC Infect Dis 24, 326 (2024). https://doi.org/10.1186/s12879-024-09212-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09212-4