Abstract
Background
Empyema necessitans (EN) is a rare condition characterized by pleural infection with pus spreading into adjacent soft tissues. Although Mycobacterium tuberculosis and Actinomyces israelii are common causative agents, methicillin-resistant Staphylococcus aureus (MRSA) is relatively rare, but it is associated with high mortality in empyema cases. We aimed to report a unique case of EN caused by MRSA and present a literature review to better understand this rare condition.
Case presentation
A 69-year-old man with a history of right ureteral stone presented with fever and left anterior thoracic pain. A physical examination revealed redness and swelling in the left thoracic region. Imaging studies confirmed EN with fluid accumulation around the sternocostal joint of the left first rib. MRSA was identified from blood and pleural fluid cultures. The patient received antimicrobial therapy, and a chest tube was inserted for drainage. Despite initial improvement, vertebral osteomyelitis was diagnosed on day 17. The antimicrobials were subsequently terminated after 6 weeks, but vertebral osteomyelitis recurred, and treatment was resumed and completed on day 215.
Conclusion
EN caused by MRSA is rare, and the literature review revealed 14 cases from human sources. Positive blood cultures were observed in 40% of cases, and metastatic infections were present in 30% of cases. Osteomyelitis was the most common type of metastatic lesion. All the patients underwent drainage. Patients with MRSA-associated EN frequently develop disseminated lesions and should therefore be carefully examined. Moreover, appropriate treatment with antibiotics and drainage is necessary for a good prognosis. Although the prognosis appeared to be favorable in our review, publication bias and treatment challenges for metastatic infections should be considered.
Similar content being viewed by others
Background
Empyema necessitans (EN) refers to an infection of the pleura and the associated spread of pus beyond the pleural cavity into adjacent soft tissue structures [1]. Mycobacterium tuberculosis (M. tuberculosis) and Actinomyces israelii (A. israelii) are the most common causative organisms of EN [2,3,4]. In recent years, the incidence of EN has decreased with the use of antibiotics [5].
Methicillin-resistant Staphylococcus aureus (MRSA) is a relatively rare causative agent of empyema. Furthermore, those associated with bacteremia have a mortality rate as high as 42.1% [6]. Based on the above, EN caused by MRSA has rarely been reported, but it is expected to be more severe. We aimed to report the case of EN caused by MRSA, and owing to its rarity, we performed a literature review to investigate its complications, management, and prognosis.
Case presentation
A 69-year-old man with an asymptomatic right ureteral stone presented to the hospital with a chief complaint of fever that had begun one week earlier. He took no oral medications, had a 100-pack-year smoking history, and consumed 350 mL/day of beer. He had no allergies or significant family history. He had worked for many years in the tuna brokering business but had retired several months earlier and was currently unemployed. Six days before his visit, the patient developed redness and pain in the left anterior thoracic region and had difficulty raising his left arm. The day before the visit, he experienced gross hematuria and was prescribed sitafloxacin at a nearby clinic for a suspected urinary tract infection. On admission, the patient was conscious, with a Glasgow Coma Scale score of E4V5M6, temperature of 38.1°C, blood pressure of 140/80 mmHg, pulse of 99/min, respiratory rate of 28/min, and oxygen saturation of 99% (nasal cannula, 1 L/min). Physical examination revealed redness, hot tenderness, fluctuant swelling, and bulging in the left anterior thoracic region (Fig. 1). Peripheral signs suggestive of infective endocarditis were observed. No crackles were heard on auscultation, and there was no spinous process tenderness. Laboratory findings revealed the following: white blood cell count, 22,700/μL (neutrophils, 90.5%; lymphocytes, 5.5%; monocytes, 3.0%) (normal range: 3,300–8,600/L); creatinine, 0.85 mg/dL (normal range: 0.65–1.07 mg/dL); total protein, 6.9 g/dL (normal range: 6.6–8.1 g/dL); lactate dehydrogenase (LDH), 269 U/L (normal range: 124–222 U/L); glucose, 162 mg/dL (normal range: 73–109 mg/dL), and C-reactive protein, 37.8 mg/dL (normal range: 0.00–0.14 mg/dL). Urinalysis revealed occult blood 2 + and leukocytes 1 + .
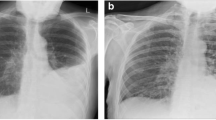
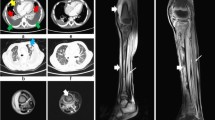
Chest radiography revealed an infiltrative shadow in the left upper lung field, and contrast-enhanced computed tomography (CT) showed fluid accumulation with marginal contrast enhancement around the sternocostal joint of the left first rib, extending subcutaneously (Fig. 2a, b). This image findings were consistent with EN. A small-bore chest tube was inserted at the same site, and purulent turbid drainage was obtained. Pleural fluid revealed a pH of 6.9, total protein of 3.9 g/dL, LDH of 3,561 U/L, glucose of 25 mg/dL, adenosine deaminase of 87.1 U/L, and total cell count of 24,900/μL (neutrophils, 98%; monocytes, 2.0%). On the same day, ampicillin/sulbactam 3 g every 6 h was started; on the second day, vancomycin (VAN) 1.25 g every 12 h was added because Gram-positive cocci in clusters were observed in the Gram stain from the blood and pleural fluid collected on admission. Acid-fast bacilli smear, culture, and polymerase chain reaction of the pleural fluid specimen were all negative. The serum trough concentration of VAN was 15–20 mg/L. On the third day, a chest radiography revealed that the infiltrative shadow in the left upper lung field was reduced; however, an infiltrative shadow in the left lower lung field appeared, and a drain was added at the site. On the fourth day, the final culture revealed MRSA in the blood and pleural fluid at the time of admission. This was confirmed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Biotyper, Bruker Daltonik GmbH, Bremen, Germany). The susceptibility test was performed using the MicroScan Walkaway Plus automatic system (Beckman Coulter, USA) (Table 1). A blood culture obtained on day 6 also showed persistent positivity; therefore, daptomycin 700 mg (9 mg/kg) was added every 24 h. Blood cultures obtained on day eight yielded negative results. Transthoracic echocardiography was performed twice, with one week interval, with no findings suggestive of infective endocarditis. On day 10, drainage from the chest tube was decreased, and the shadows on the chest radiograph improved; therefore, the chest tube was removed. Thereafter, the fever resolved; however, on the 17th day, the patient had fever with neck pain, and contrast-enhanced magnetic resonance imaging (MRI) revealed contrast enhancement of the vertebral body and perivertebral space at C7–T1, which led to the diagnosis of vertebral osteomyelitis. No epidural abscess was observed. The patient clinically improved and was discharged from the hospital on the 28th day because the fever gradually resolved, cervical pain tended to improve, and the antimicrobial agent was changed to oral linezolid 600 mg every 12 h.
Chest radiography and contrast-enhanced chest computed tomography scan acquired on admission. a Chest radiography reveals an infiltrative shadow in the left upper lung field. b Contrast-enhanced computed tomography reveals fluid accumulation with marginal contrast enhancement around the sternocostal joint of the left first rib, extending subcutaneously
Taste disturbance due to linezolid was observed; however, chest radiography revealed a decrease in pleural effusion, and the treatment was terminated on day 58. In retrospect, the erythrocyte sedimentation rate (ESR) at this time was 80 mm/h. On day 67, the patient again presented with neck pain and fever, and contrast-enhanced CT revealed enhanced soft tissue shadows around the C7–T1 vertebral body. He was readmitted with a diagnosis of a flare-up of vertebral osteomyelitis. Therefore, we restated VAN. There was no worsening of pleural effusion on chest radiography. The patient continued VAN for 14 days and was then switched to oral sulfamethoxazole–trimethoprim (SXT) 160 mg/800 mg every 12 h. On day 125, due to elevated liver enzyme levels, the patient was administered daptomycin for three days. The enzyme levels quickly normalized and were elevated only once during this period. Subsequently, the treatment was switched to oral minocycline 100 mg every 12 h. After confirming that the ESR had normalized, treatment was terminated on day 215. No relapse has occurred since then.
Methods of literature review
Two authors independently reviewed the titles and abstracts of the database records, retrieved the full texts for eligibility assessment, and extracted data from the case reports. We searched for case reports of empyema due to MRSA and reviewed the images individually to determine if they qualified for EN. We searched the PubMed and Embase databases using specific keywords (Additional file 1). The following filters were applied: English or Japanese language and articles registered in the literature database until April 30, 2023. Conference abstracts were excluded. The PubMed and Embase searches generated 265 and 518 articles, respectively. Of these, 259 and 511 reports from PubMed and Embase, respectively, were excluded since either they were not case reports or case reports that did not focused on EN based on the images included. Considering that there were several reports in Japanese papers, we included only those published in Japanese to further understand the clinical characteristics of the disease by presenting more confirmed cases. To search for articles in Japanese, we used Ichushi-Web, a major Japanese database, using some keywords (Additional file 1). We examined the eligibility and the work conducted in PubMed and Embase, and finally, one case was included. We searched Google Scholar and identified five additional human cases. Finally, we reviewed 13 articles that included 14 human cases (Fig. 3, Table 2).
Discussion and conclusion
EN is a rare clinical condition that was first described by Gullan De Baillon in 1640; it refers to an infection of the pleura and the associated spread of pus beyond the pleural cavity into adjacent soft tissue structures [5]. EN typically results from necrotizing pneumonia that has been present for a long period and may also occur after other trauma or open chest surgery [18]. M. tuberculosis and A. israelii are usually identified from pleural effusions as the main causative agents [2,3,4]. However, EN caused by MRSA is rare. Although there are reports of an overall mortality rate of 66% in the pre-antimicrobial era of EN [1], limited information is available [6]. Based on the above, EN caused by MRSA has rarely been reported, but is expected to be more severe. We reported the case of EN caused by MRSA, and owing to its rarity, we performed a literature review to investigate its complications, management, and prognosis.
Fifteen patients, including the present patient, were included in the literature review. The patients consisted of six adults (median age: 59 years, range: 35–69 years) and nine children (median age, 1.5 years, range: 4 weeks–10 years), with a slightly larger proportion of males (n = 10, 67%). The proportions of underlying diseases and risk factors were lower in children (n = 3, 33%) and higher in adults (n = 5, 83%). Factors such as liver cirrhosis (n = 2), postoperative state (n = 2), and diabetes mellitus (n = 1) were identified in the adult patients. Blood culture positivity was present in 40% (n = 8) and disseminated lesions were present in 30% (n = 6) of the patients. Disseminated lesions included three cases of osteomyelitis of the ribs due to direct deep penetration of the empyema, two cases of osteomyelitis as metastatic lesions, and one case each of multiple intramuscular abscesses and septic pulmonary embolism. MRSA infections cause metastatic infections [19]. It is particularly important to identify metastatic infections when blood cultures are positive, as in the present case [20]. Osteomyelitis, especially in our literature review, is frequently reported and should be considered for diagnosis using MRI if suspected.
Regarding surgical interventions, 73% (n = 11) had a tube thoracostomy, 33% (n = 5) an approach to a subcutaneous abscess, 27% (n = 4) thoracoscopic decortication, 20% (n = 3) a partial lung resection, and one patient each had used an intrapleural fibrinolytic agent, open chest surgery, continuous intrathoracic irrigation, and removal of the prosthesis. All patients underwent some form of surgical intervention, and none received antimicrobial therapy alone. Regarding the prognosis, no death cases were reported. A high mortality rate for MRSA empyema has been described in previous reports; however, there is no description of its actual treatment [6]. In all cases in this review, appropriate surgical intervention was performed, which may have led to a better prognosis. We should consider the aggressive drainage with reference to the treatment performed in this literature review. Furthermore, there has been only one case of treatment failure in the literature review, which was our case. In our case, the disseminated lesion was vertebral osteomyelitis, and MRSA osteomyelitis has a high relapse rate, with approximately 30% of patients relapsing after less than eight weeks of treatment [21]. Therefore, anti-MRSA agents were initially administered in this case for eight weeks. However, in retrospect, the patient had a high ESR of 80 mm/h at eight weeks and may have been at high risk of recurrence. Even with the appropriate treatment for empyema, some metastatic infection sites can be difficult to treat, and clinicians should pay close attention when treating metastatic infection.
In this case, daptomycin was added after confirming persistent bacteremia during the VAN continuation. Therapeutic drug monitoring (TDM) with VAN is recommended with a ratio of area under the curve over 24 h to minimum inhibitory concentration (AUC/MIC) of ≥ 400 [22]. Since trough concentrations in the range of 15–20 μg/mL may be compatible with an AUC/MIC of ≥ 400 [23], we implemented trough-guided TDM according to the available resources at our hospital. VAN is widely prescribed and the first choice of treatment for MRSA bacteremia [24]. However, several disadvantages associated with VAN administration have been reported, including low tissue penetration, slow bactericidal effect, and the emergence of resistant strains during treatment [25]. In our case, although we maintained an optimal trough for VAN, we were unable to achieve a negative conversion of MRSA bacteremia. In recent years, several reports have demonstrated a significant reduction in 30-day mortality when daptomycin was initiated within 72 h of the onset of MRSA bacteremia [26,27,28]. In addition, daptomycin is associated with good tissue transfer, and the successful treatment of empyema by switching from linezolid to VAN has been reported in some cases [29, 30]. According to some reports, daptomycin is less likely than VAN to cause clinical failure [31]. In the case of MRSA, which has a high mortality rate, studies are being conducted using a combination of anti-MRSA drugs and the beta-lactam antibiotics SXT and fosfomycin; however, good results in terms of patient outcomes have not been obtained. With the combination of daptomycin and VAN, some older studies have demonstrated that all Staphylococcus aureus isolates develop daptomycin nonsusceptibility in the presence of VAN [32]. Based on the results of this study, there have been few studies on combined therapy with daptomycin and VAN. However, there is a lack of clear evidence on the deterioration associated with combined therapy [33, 34]. Daptomycin cannot be used for microorganisms via the alveoli because it is inactivated by a type 2 surfactant; however, it can be used effectively for empyema. Therefore, we believe that a combination of VAN and daptomycin can be used effectively and safely to treat empyema.
In EN as a whole, several cases were reported in the preantibiotic era, and in recent years, the number of cases has been declining, regardless of the bacterial species [35]. It has been suggested that this is due to the fact that in most cases of infection, patients respond quickly to antimicrobial agents when properly diagnosed [36]. Therefore, the diagnosis of EN in the modern era may be the result of delayed diagnosis or severe cases. A review of the period 1966–2004, when antimicrobial agents were widely used, revealed that M. tuberculosis and A. israelii were the most common causative organisms [1]. In contrast, a 2010 review noted an increase in the frequency of MRSA as a cause [36]. In our review, 66% (10/15) of the cases occurred from 2010 onward. In summary, even the usual MRSA empyema is difficult to treat in the first place, while MRSA EN is likely to be even more severe. Recently, the severity of EN as a disease has increased. The importance of MRSA EN as a disease has increased as well in recent years.
As discussed above, compared to usual empyema, the causative pathogens of EN are more frequently represented by MRSA or tuberculosis, even in the community-acquired infections. These causative pathogens cannot be eradicated by empirical therapy alone, which is commonly used to treat empyema [2,3,4, 37]. Therefore, identifying the causative organism is more important in the EN than in usual empyema. Drainage should be performed first when EN is suspected, both for therapeutic purposes and to identify the causative organism. In the present case, drainage was performed on the day the patient arrived for prompt diagnosis and appropriate treatment.
In conclusion, we encountered a rare case of empyema caused by MRSA. Early drainage of the empyema should be performed to identify the causative pathogen and develop an optimal management strategy.
Availability of data and materials
Not applicable.
Abbreviations
- AUC:
-
Area Under the Curve
- CT:
-
Computed Tomography
- EN:
-
Empyema Necessitans
- ESR:
-
Erythrocyte Sedimentation Rate
- LDH:
-
Lactate Dehydrogenase
- MIC:
-
Minimum Inhibitory Concentration
- MRI:
-
Magnetic Resonance Imaging
- MRSA:
-
Methicillin-Resistant Staphylococcus aureus
- SXT:
-
Sulfamethoxazole–Trimethoprim
- TDM:
-
Therapeutic Drug Monitoring
- VAN:
-
Vancomycin
References
Freeman AF, Ben-Ami T, Shulman ST. Streptococcus pneumoniae empyema necessitatis. Pediatr Infect Dis J. 2004;23:177–9.
Akgül AG, Örki A, Örki T, Yüksel M, Arman B. Approach to Empyema Necessitatis. World J Surg. 2011;35:981–4.
Stallworth J, Mack E, Ozimek C. Methicillin-resistant Staphylococcus aureus Empyema Necessitatis in an Eight-month-old Child. South Med J. 2005;98:1130–1.
Tonna I, Conlon CP, Davies RJO. A case of empyema necessitatis. Eur J Intern Med. 2007;18:441–2.
Mizell KN, Patterson KV, Carter JE. Empyema necessitatis due to methicillin-resistant Staphylococcus aureus : Case report and review of the literature. J Clin Microbiol. 2008;46:3534–6.
Turnidge JD, Kotsanas D, Munckhof W, Roberts S, Bennett CM, Nimmo GR, et al. Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust. 2009;191:368–73.
Kugai T, Koja K, Kuniyoshi Y, Iha K, Akasaki M, Miyagi K, et al. Methicillin-resistant Staphylococcus aureus pyothorax following radical correction of Budd-Chiari Syndrome. Ryukyu daigaku igakukai zasshi Ryukyu Med J. 1993;13:97–103.
Moore FO, Berne JD, McGovern TM, Ravishankar S, Slamon NB, Hertzog JH. Empyema necessitatis in an infant: a rare surgical disease. J Pediatr Surg. 2006;41:e5-7.
Contreras GA, Pérez N, Murphy JR, Cleary TG, Heresi GP. Empyema necessitans and acute osteomyelitis associated with community-acquired methicillin-resistant Staphylococcus aureus in an infant. Biomedica. 2009;29:506–12.
Rosebush J, Summers R, Snitzer J, Spearman P, Jerris R, Satola S. Methicillin-resistant Staphylococcus aureus empyema necessitatis in a breast-fed neonate. Pediatr Infect Dis J. 2014;33:668–9.
Lee HC, Li CL, Liao DL, Lee WJ, Tsai CM, Niu CK, et al. Community-associated Methicillin-resistant Staphylococcus aureus empyema necessitatis in a 1-year-8-month-old child. Official bull Soc Pediatr Pulmonol Taiwan, ROC. 2015;11:48–51.
Edriss H, Berdine G. Empyema necessitatis secondary to Staphylococcus aureus lung abscess. Southwest Respir Critical Care Chronicles. 2017;5:58.
Pugh CP. Empyema necessitans a rare complication of methicillin-resistant Staphylococcus Aureus empyema in a child. Pediatr Infect Dis J. 2020;39:256–7.
Wagh P, Rajpurohit R. Right lateral chest wall empyema necessitans secondary to lung abscess. J Datta Meghe Inst Med Sci Univ. 2021;16:792.
Farouji I, Halabiya M, Battah A, Miller RA. Methicillin-Resistant Staphylococcus Aureus: A Rare Cause of Empyema Necessitatis. Am J Respir Crit Care Med. 2021;203:A3972.
Ashraf S, Masood S, Meer T, Iqbal S, Rashid J. Empyema necessitans caused by methicillin resistant Staphylococcus Aureus – Two rare presentations. Pak Pediatr J. 2022;46:224–8.
Rehman AU, Iqbal R, Siddiqui A, Khan S, Ahmad W, Rehman Siddiqui NU. Empyema necessitans secondary to methicillin resistant Staphylococcus aureus, a rare complication of empyema. EC Paediatrics. 2023;12:41–6.
Ahmed SI, Gripaldo RE, Alao OA. Empyema necessitans in the setting of pneumonia and parapneumonic effusion. Am J Med Sci. 2007;333:106–8.
Horino T, Hori S. Metastatic infection during Staphylococcus aureus bacteremia. J Infect Chemother. 2020;26:162–9.
Parsons JB, Westgeest AC, Conlon BP, Fowler VG. Persistent methicillin-resistant Staphylococcus aureus Bacteremia: Host, pathogen, and treatment. Antibiotics. 2023;12:455.
Park KH, Cho OH, Lee JH, Park JS, Ryu KN, Park SY, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis. 2016;62:1262–9.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77:835–64.
Clark L, Skrupky LP, Servais R, Brummitt CF, Dilworth TJ. Examining the relationship between vancomycin area under the concentration time curve and serum trough levels in adults with presumed or documented Staphylococcal infections. Ther Drug Monit. 2019;41:483–8.
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clin Infect Dis. 2011;52:e18-55.
Davis J, Hal S, Tong S. Combination antibiotic treatment of serious methicillin-resistant Staphylococcus aureus infections. Semin Respir Crit Care Med. 2015;36:003–16.
Schweizer ML, Richardson K, Vaughan Sarrazin MS, Goto M, Livorsi DJ, Nair R, et al. Comparative Effectiveness of Switching to Daptomycin Versus Remaining on Vancomycin Among Patients With Methicillin-resistant Staphylococcus aureus (MRSA) Bloodstream Infections. Clin Infect Dis. 2021;72:S68-73.
Murray KP, Zhao JJ, Davis SL, Kullar R, Kaye KS, Lephart P, et al. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: A matched cohort study. Clin Infect Dis. 2013;56:1562–9.
Claeys KC, Zasowski EJ, Casapao AM, Lagnf AM, Nagel JL, Nguyen CT, et al. Daptomycin Improves Outcomes Regardless of Vancomycin MIC in a Propensity-Matched Analysis of Methicillin-Resistant Staphylococcus aureus Bloodstream Infections. Antimicrob Agents Chemother. 2016;60:5841–8.
Yagi Y, Iizuka M, Okazaki M, Jobu K, Morita Y, Miyamura M. Daptomycin for the successful treatment of postoperative methicillin-resistant Staphylococcus aureus empyema: a case report. J Chemother. 2021;33:431–4.
Torjani A, Selbst D, Hamsher J, Mujumdar S, Belkoff A, Taboada L. Successful treatment with daptomycin of MRSA empyema complicated by right-sided loculated pleural effusion refractory to vancomycin. Clin Med Insights Case Rep. 2022;15:117954762210785.
Maraolo AE, Giaccone A, Gentile I, Saracino A, Bavaro DF. Daptomycin versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus bloodstream infection with or without endocarditis: A Systematic review and meta-analysis. Antibiotics. 2021;10:1014.
Sakoulas G, Alder J, Thauvin-Eliopoulos C, Moellering RC, Eliopoulos GM. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob Agents Chemother. 2006;50:1581–5.
Tsuji BT, Rybak MJ. Etest synergy testing of clinical isolates of Staphylococcus aureus demonstrating heterogeneous resistance to vancomycin. Diagn Microbiol Infect Dis. 2006;54:73–7.
Antony SJ. Combination therapy with daptomycin, vancomycin, and rifampin for recurrent, severe bone and prosthetic joint infections involving methicillin-resistant Staphylococcus aureus. Scand J Infect Dis. 2006;38:293–5.
Kono SA, Nauser TD. Contemporary Empyema Necessitatis. Am J Med. 2007;120:303–5.
Llamas-Velasco M, Domínguez I, Ovejero E, Pérez-Gala S, García-Diez A. Empyema necessitatis revisited. Eur J Dermatol. 2010;20:115–9.
Maskell NA, Batt S, Hedley EL, Davies CWH, Gillespie SH, Davies RJO. The Bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am J Respir Crit Care Med. 2006;174:817–23.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
No funding was received for the conception, composition, editing, or submission of this manuscript.
Author information
Authors and Affiliations
Contributions
The manuscript was seen and approved by all the authors and is not under consideration for publication elsewhere. All the authors contributed to this work. TN collected clinical data and wrote the initial draft of the manuscript. TN, NM, and KS performed the review of the literature. KI, NK, NM, and TJ supervised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and the accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search terms used to search three databases (PubMed, Embase, and Ichushi) for literature reviews on empyema necessitans associated with MRSA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nakamura, T., Ishikawa, K., Murata, N. et al. Empyema necessitans caused by methicillin-resistant Staphylococcus aureus: a case report and literature review. BMC Infect Dis 24, 157 (2024). https://doi.org/10.1186/s12879-024-09062-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09062-0