Abstract
The pathological consequences of inflammation persist in people living with the human immunodeficiency virus (PLWH), regardless of the positive outcomes of highly active antiretroviral therapy (HAART). The current systematic review and meta-analysis aims to understand and explore the levels of high-sensitivity C-reactive protein (hs-CRP) and other cardiovascular disease (CVD)-risk factors including lipid profiles among PLWH on HAART. Major electronic databases including PubMed, Scopus, and Web of Science were searched to retrieve relevant global literature reporting on hs-CRP levels in PLWH on HAART. A total of twenty-two studies with an average participant age of 40 years were eligible for this systematic review and meta-analysis. Majority of the included studies were from Africa (n = 11), the United States (n = 6), and Europe (n = 5). Our systemic review showed that most studies reported increased levels of hs-CRP among PLWH on HAART when compared to controls (PLWH not on HAART or those without HIV), especially in studies from Africa. This was supported by a meta-analysis showing significantly elevated levels of hs-CRP in PLWH on HAART when compared to PLWH not on HAART (standardised mean difference [SMD] = 0.56; 95% CI = 0.10‑1.01, z = 2.41; p = 0.02) or those without HIV (SMD = 1.19; 95% CI = 0.76‑1.63, z = 5.35; p < 0.001). Where lipid profiles, as a major predictor for CVD risk, were also impaired in PLWH on HAART when compared to PLWH not on HAART and HIV-negative participants. In conclusion, elevated levels of hs-CRP and lipid levels are prevalent in PLWH on HAART, this may increase the risk of CVD complications, especially for those people living in Africa. However, more evidence in larger population studies is required to confirm these outcomes and unveil any possible clinical implications of HAART-induced modulation of hs-CRP levels in PLWH.
Similar content being viewed by others
Introduction
The human immunodeficiency virus (HIV) is a persistent public health problem that currently affects approximately 36.9 million individuals worldwide [1]. This pandemic has grave economic implications, especially in high-prevalence regions such as sub-Saharan Africa (SSA) [2]. Sub-Saharan Africa remains the epicentre for the HIV pandemic, accounting for more than 70% of the global infected population [3]. However, the availability of highly-active antiretroviral therapy (HAART) has been widely acknowledged for its effectiveness in significantly improving life expectancy and quality of life among people living with HIV (PLWH) [4,5,6]. Despite these improvements, prolonged use of HAART has been associated with other comorbidities (diabetes mellitus, hypertension, and dyslipidemia) that may lead to the development of cardiovascular diseases (CVDs) [7, 8]. Noteworthy, PLWH on long-term HAART are predicted to be two times more likely to develop CVDs compared to those without this condition [9], whilst the proportion of deaths attributed to CVDs in PLWH on HAART has doubled in the last decade [6, 8]. This knowledge has shifted the focus of care for PLWH, and it now calls for a better understanding of the disease pathophysiology, which makes it necessary to devise new intervention strategies to combat CVD-related complications.
Traditional risk factors and comorbidities such as diabetes mellitus, hypertension, and dyslipidemia are known to be associated with the development and progression of CVDs [10, 11]. These factors are often accompanied by inflammation, a pathological hallmark for HIV infection and CVDs [12]. Although inflammation is necessary for an adequate immune response, a dynamic balance must be achieved between pro- and anti-inflammatory factors in order to suppress infection and minimize any metabolic complications [13, 14]. Beyond their involvement in driving undesired immune activation [15], the most commonly studied pro-inflammatory markers with regard to the pathogenesis of HIV and CVD include interleukin 6 (IL-6), tumor necrosis factor (TNF-α), and high-sensitivity C-reactive protein (hs-CRP). These markers are, in part, associated with the extended use of HAART [16, 17]. Having previously been considered a traditional marker of infection and cardiovascular events [18], hs-CRP is now acknowledged for its role in the underlying inflammatory processes. This includes its activation of other pro-inflammatory cytokines such IL-6 and TNF-α [19].
As such, persistently elevated levels of hs-CRP are deemed to be among the reliable predictors of CVDs in PLWH on HAART [20,21,22,23,24,25]. However, other researchers have not seen this effect in PLWH on HAART [26, 27]. Several factors can contribute to the negative association in PLWH, including high lipid profiles, ethnicity, geographical location, and duration of treatment with HAART [24, 26]. Besides updating the status of clinical evidence on the role of this pro-inflammatory marker in PLWH, it remains essential to establish or generate data to evaluate whether hs-CRP levels may be a reliable biomarker to predict CVD risk in PLWH on HAART. Perhaps highlighting the significance of the current systematic review and meta-analysis to assess the levels of hs-CRP in relation to the manifestation of CVDs in PLWH on HAART.
Methods
Search strategy
A complete global literature search for publications dating from 1996 (after the introduction of HAART [28]) until August 2023 was conducted using medical subject headings (MeSH) including “C-reactive protein”, “CRP”, “cardiovascular disease”, “CVD”, “human immunodeficiency virus”, “HIV”, “inflammation”, “ART”, “antiretroviral therapy” and “highly active antiretroviral therapy” following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [29]. The search strategy that was applied is attached as Table S1. The search was done thoroughly by two independent investigators (SEM and KM). Databases searched included PubMed, Scopus, and Web of Science to identify all relevant articles. A manual search on Google Scholar was also done to identify any extra studies and to identify grey literature, especially data from conference proceedings. The meta-analysis was not registered with the Prospective Register of Systematic Reviews (PROSPERO), however caution was taken not to duplicate any existing systematic review and meta-analysis on hs-CRP.
Inclusion criteria and data extraction
Studies were included if they met the following criteria: (a) observational studies and clinical trials; (b) evaluated the modulation of the inflammatory marker hs-CRP in PLWH on HAART. Studies were excluded if (a) they were conducted before the introduction of HAART (1996), (b) they were nonhuman studies or (c) reviews. The current review and meta-analysis applied the following PECO (population, exposure, control, and outcomes):
-
Participants: PLWH on HAART.
-
Exposure: PLWH receiving any form of HAART regimen.
-
Control: PLWH not on HAART and HIV-negative participants.
-
Outcome: Hs-CRP levels and CVD-related outcomes.
Data extraction
The extracted data was independently and carefully assessed for compliance with the inclusion or exclusion criteria by three authors who resolved disagreements by consensus. The following information was extracted from each study: the first author, publication year, country, ethnicity, sample size, mean age, treatment duration, and key findings. Language restrictions were not applied during the search, however, studies conducted in other languages that could not be translated into English were excluded. The American Heart Association and Centre for Disease Control classification of cardiovascular risk according to hs-CRP level were used. For example, a hs-CRP level of > 3mg/L represents a high risk, 1-3mg/L intermediate risk, and < 1 mg/L low risk for CVD in humans [25, 30].
Quality assessment
For studies incorporated in the current systematic review and meta-analysis, the quality of evidence and risk of bias assessment was evaluated using the modified Downs and Black checklist, which rates studies out of 27 questions [31]. The Downs and Black checklist assesses five domains to determine the quality of the study, this includes: reporting bias, external validity, internal validity, selection bias, and power. The quality of evidence and risk of bias was based on evidence reported in the full-text article deemed eligible for inclusion in this systematic review and meta-analysis. Two independent investigators assessed the quality of evidence and the risk of bias for the eligible studies. Disagreements among investigators were resolved by consulting a third independent investigator.
Statistical analysis
The effect size for continuous data across all outcomes was determined by calculating the mean, standard deviation (SD), and sample size for each study. In instances where the included study only reported the mean, the SD was computed using the interquartile range (IQR) [32]. The meta-analysis results are presented as forest plots for all outcome measures, and the pooled Standard Mean Difference (SMD) was calculated using the random effects model meta-analysis. Statistical analysis considered I2 < 25%, 25–75%, and > 75% for minimal, moderate, and extreme heterogeneity, respectively [33]. All analyses were performed using Review Manager version 5.4.1 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2020).
Results
Characteristics of included studies
Our systematic search strategy identified a total of 1337 relevant records (Fig. 1). However, the final screening process yielded 22 eligible publications, that reported on hs-CRP levels in PLWH on HAART. In terms of region, most included studies were from Africa (n = 11), followed by the United States (n = 6), whilst the remaining literature was from Europe (n = 5). Included studies were published between the years 2004 to 2020, with the included participants having an average of 40 years. Included studies contained varied sample sizes for PLWH on HAART, ranging from the highest (n = 170) monitored for 9 months [34], and the smallest number of PLWH on HAART (n = 19) monitored for 24 months [35]. The majority of studies in the review used blood serum samples (n = 16) rather than plasma (n = 6) to analyze the hs-CRP levels.
Qualitative analysis of included literature
The included studies were predominantly from the African continent, especially countries from sub-Saharan Africa. Furthermore, most of the studies (n = 11) showed that serum levels of hs-CRP were significantly elevated in PLWH on HAART (Table 1). Some of these studies indicated that hs-CRP may occur concurrently with other CVD-related complications as elevated levels of this pro-inflammatory maker were consistent with increased systolic blood pressure and abnormal lipid profiles [26, 36]. Increased systolic blood pressure [36] and abnormal lipid profiles, including elevated levels of total cholesterol (TC), low-density lipoprotein (LDL)-c, and triglycerides are well-established indicators of CVD-risk [37, 38]. It was interesting to see that waist circumference [39], and other pro-inflammatory markers such as tumor necrosis factor (TNF)-α were persistently high in PLWH on HAART [40], in studies based in Africa. In fact, it was interesting to observe that hs-CRP levels remained persistently high in PLWH taking the preferred first-line HAART regimen (Stavudine: d4T, Lamivudine: 3TC, and Efavirenz: EFZ) [41] regardless of study duration. This likely indicates that immune activation and inflammation persist in PLWH regardless of viral suppression, as previously discussed [42]. Also disputing the fact that ethnicity and environmental factors could play a major role in driving these pathological features, as some studies from the United States (n = 6) and Europe (n = 5) showed conflicting results in terms of hs-CRP levels and coronary heart disease (CHD) outcomes in PLWH on HAART when compared to those from Africa.
Quantitative analysis of included literature
Circulating levels of hs-CRP in PLWH on HAART in comparison to PLWH not on HAART
Firstly, we analyzed the levels of hs-CRP in PLWH on HAART versus PLWH not on HAART (Fig. 2). The quantitative (pooled) analysis of twelve included studies showed that hs-CRP levels were significantly increased in PLWH on HAART when compared to PLWH not on HAART (SMD = 0.56; 95% CI = 0.10 ‑1.01, z = 2.41; p = 0.02). The subgroup analysis, based on the region where the study was performed, revealed no association; however, elevated levels of hs-CRP favoured studies from Africa (SMD = 0.80; 95% CI = -0.01‑ 1.61, z = 1.93; p = 0.05), Europe (SMD = 0.27; 95% CI = -0.25 ‑ 0.80, z = 1.03; p = 0.31) and the United States (SMD = 0.34; 95% CI = -0.00 ‑ 0.68, z = 1.93; p = 0.05). However, the tests for subgroup differences showed no statistically significant differences (p = 0.54) with 0% of heterogeneity (Fig. 2). Suggesting that geographical region does not influence hs-CRP levels in PLWH on HAART versus PLWH not on HAART.
A forest plot showing outcomes of a meta-analysis for high sensitivity C-reactive protein (hs-CRP) levels in people living with the human immunodeficiency virus (PLWH) on highly active antiretroviral therapy (HAART), compared to PLWH not on HAART. Green squares represent the weight of each study in the average effect size. Horizontal lines across green squares represent the 95% confidence intervals for the point estimate. The diamonds represent the weighted average point estimate
Circulating levels of hs-CRP in PLWH on HAART in comparison to individuals without HIV
The second aim of this meta-analysis, utilizing pooled data, was to evaluate whether HAART treatment affects hs-CRP levels in PLWH when compared to uninfected individuals (Fig. 3). The quantitative (pooled) analysis of eight included studies showed that hs-CRP levels were significantly increased in PLWH on HAART, in comparison to individuals without HIV (SMD = 1.19; 95% CI = 0.76 ‑ 1.63, z = 5.35; p < 0.001). The performed subgroup analysis showed no statistical significance between subgroups (p = 0.57) with 0% heterogeneity. Suggesting that geographical region does not influence hs-CRP levels in PLWH on HAART versus HIV-negative control. However, there was a significant increase in hs-CRP levels in PLWH on HAART when compared to negative controls in studies from Africa (SMD = 1.31; 95% CI = 0.55 ‑ 2.07, z = 3.38; p = 0.0007), Europe (SMD = 1.37; 95% CI = 0.76 ‑ 1.98, z = 4.39; p < 0.0001) and United States (SMD = 0.98; 95% CI = 0.52 ‑ 1.45, z = 4.13; p < 0.001).
A forest plot showing outcomes of a meta-analysis for high sensitivity C-reactive protein (hs-CRP) levels in people living with the human immunodeficiency virus (PLWH) on high active antiretroviral therapy (HAART), compared to HIV-negative controls. Green squares represent the weight of each study in the average effect size. Horizontal lines across green squares represent the 95% confidence intervals for the point estimate. The diamonds represent the weighted average point estimate
Circulating levels of hs-CRP in PLWH not on HAART in comparison to individuals without HIV
The third objective of this meta-analysis of pooled data was to assess whether HIV infection increases the levels of hs-CRP in PLWH not on HAART when compared with uninfected individuals (Fig. 4). The overall analysis showed that hs-CRP levels were significantly increased in PLWH not on HAART, in comparison to individuals without HIV (SMD = 0.85; 95% CI = 0.45‑1.26, z = 4.11; p < 0.0001). There was a significant association between PLWH not on HAART and elevated levels of hs-CRP in studies conducted in Africa (SMD = 0.66; 95% CI = 0.05‑1.28, z = 2.11; p = 0.03), Europe (SMD = 2.23; 95% CI = 1.52‑2.93, z = 6.17; p < 0.0001) and the United States (SMD = 0.75; 95% CI = 0.40‑1.11, z = 4.16; p < 0.0001).
A forest plot shows meta-analysis outcomes for high sensitive C-reactive protein (hs-CRP) levels in people living with the human immunodeficiency virus (PLWH) not on highly active antiretroviral therapy (HAART), compared to HIV-negative controls. Green squares represent the weight of each study in the average effect size. Horizontal lines across green squares represent the 95% confidence intervals for the point estimate. The diamonds represent the weighted average point estimate
Lipid profiles in PLWH on HAART in comparison to PLWH not on HAART
We conducted an analysis of lipid profile levels, encompassing of HDL-c, LDL-c, total cholesterol, and triglycerides, in PLWH on HAART in comparison to PLWH not on HAART (Fig. 5). Specific analyses revealed a non-significant effect on HDL-c (SMD = 0.22; 95% CI = -0.29 ‑ 0.74, z = 0.84; p = 0.40), but significantly increased LDL-c (SMD = 1.32; 95% CI = 0.57 ‑ 2.08, z = 3.44; p = 0.0006), total cholesterol (SMD = 1.34; 95% CI = 0.21 ‑ 2.48, z = 2.32; p = 0.02), and triglyceride (SMD = 1.25; 95% CI = 0.55—1.96 z = 3.48; p < 0.0005) levels in PLWH on HAART compared to PLWH not on HAART. Notably, there was a substantial level of heterogeneity, exceeding 95%, in these lipid profiles.
A forest plot showing meta-analysis outcomes for lipid profiles, including high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), total cholesterol (TC) and triglycerides (TG), in people living with the human immunodeficiency virus (PLWH) on highly active antiretroviral therapy (HAART) in comparison to PLWH not on HAART. Green squares represent the weight of each study in the average effect size. Horizontal lines across red squares represent the 95% confidence intervals for the point estimate. The diamonds represent the weighted average point estimate
Lipid profiles in PLWH on HAART in comparison to individuals without HIV
Lipid profiles including levels of HDL-c, LDL-c, total cholesterol, and triglycerides, were also analyzed in PLWH on HAART when compared to HIV-negative controls (Fig. 6). The specific analysis of lipid profiles showing that HDL-c was significantly reduced (SMD = -1.30; 95% CI = -2.07 ‑ 0.54, z = 3.33; p < 0.0009) while LDL-c (SMD = 1.03; 95% CI = 0.04 ‑ 2.02, z = 2.05; p < 0.04) total cholesterol (SMD = 0.51; 95% CI = 0.16 ‑ 0.87, z = 2.84; p < 0.005) and triglycerides (SMD = 1.52; 95% CI = 0.42 ‑ 2.62, z = 2.72; p < 0.007) were significantly increased in PLWH on HAART in comparison to HIV-negative controls (Fig. 6). It is worth mentioning that these studies showed a substantial level of heterogeneity (I2 = 96%).
A forest plot showing outcomes of a meta-analysis for lipid profiles, including high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), total cholesterol (TC) and triglycerides (TG), in people living with the human immunodeficiency virus (PLWH) on highly active antiretroviral therapy (HAART) in comparison to HIV-negative controls. Green squares represent the weight of each study in the average effect size. Horizontal lines across red squares represent the 95% confidence intervals for the point estimate. The diamonds represent the weighted average point estimate
Lipid profiles in PLWH not on HAART in comparison to individuals without HIV
Lastly, this meta-analysis of pooled data aimed to assess whether HIV infection increases the levels of lipid profiles in PLWH not on HAART when compared with uninfected individuals as shown in the forest plot (Fig. 7). The pooled effect estimates displayed reduced HDL-c levels in HIV participants not on HAART when compared to HIV negative individuals (SMD = -1.09; 95% CI = -1.66- ‑0.52, z = 3.77; p = 0.0002). However, these studies showed a substantial level of heterogeneity (I2 = 93%). In addition, no association in the LDL-c (SMD = 0.32; 95% CI = -0.79‑1.43, z = 0.57; p = 0.57; I2 = 98%), TC (SMD = -0.28; 95% CI = -0.59‑0.02, z = 1.81; p = 0.07; I2 = 61%), TG (SMD = 0.14.; 95% CI = 0.55 ‑0.82, z = 0.39; p = 0.70; I2 = 95%) levels, was observed in PLWH not on HAART when compared to HIV-negative individuals.
A forest plot showing outcomes of a meta-analysis for lipid profiles, including high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), total cholesterol (TC) and triglycerides (TG), in people living with the human immunodeficiency virus (PLWH) not on highly active antiretroviral therapy (HAART) in comparison to negative controls or individuals without HIV. Green squares represent the weight of each study in the average effect size. Horizontal lines across red squares represent the 95% confidence intervals for the point estimate. The diamonds represent the weighted average point estimate
Discussion
Significantly contributing to the global disease burden, CVD have become a leading cause of morbidity and mortality for PLWH in the era of effective HAART [28]. It has therefore become imperative to understand the pathogenesis of CVD within PLWH, including relevant biomarkers that drive a pro-inflammatory response like hs-CRP [25]. In fact, it has been reported that hs-CRP may be a useful biomarker associated with the development of CVD-related complications in PLWH on HAART [24, 25, 58]. Despite updating the status of clinical evidence on the relevance of hs-CRP levels in PLWH, this review aimed to establish whether this pro-inflammatory marker is modulated independently in those on HAART. Importantly, beyond reporting on the levels of hs-CRP, the current review analyzed evidence on CVD-related outcomes, including lipid profiles to predict CVD risk in PLWH on HAART. To comprehend the potential influence of ethnicity and geographic locations, clinical data was additionally examined based on the world regions classified by the country where it was published. Both the systematic approach and meta-analysis were done to strengthen the reported data.
Twenty-two studies qualified for inclusion in this systematic review, with most studies (n = 11) coming from the African region [27, 34, 36, 38, 43,44,45,46,47,48]. The latter was expected since the sub-Saharan African region remains the epicenter of the Human immunodeficiency virus pandemic, with skyrocketing infections [3, 59]. In fact, the sub-Saharan African region is estimated to overtake high-income countries with an increased burden of noncommunicable diseases, particularly due to coronary artery disease and stroke [60, 61]. With increasing research being channeled into understanding the pathogenesis of HIV and associated complications that could be implicated in driving the development of CVD, especially the involvement of inflammation [7, 21]. Consistent with this notion, the qualitative analysis of data presented in this review clearly showed that hs-CRP levels in PLWH on HAART are significantly increased when compared to PLWH not on HAART or those without HIV (Table 1). This evidence was supported by a meta-analysis showing significantly elevated levels of hs-CRP in PLWH on HAART when compared to controls (Figs. 2 and 3). With most studies supporting this increase of hs-CRP in studies from Africa, when compared to both Europe and the United States. Further exploration using subgroup analysis did not indicate any apparent differences in statistical values, suggesting that more studies are required to assess the influence of geographical region on hs-CRP levels in PLWH on HAART. In fact, these findings that support raised levels of hs-CRP in PLWH on HAART were consistent regardless of treatment duration and were not influenced by the type of HAART regimen. This indicates that inflammation is a predominant feature in PLWH regardless of viral suppression. This hypothesis has been increasingly explored recently [21, 42], with more evidence required to understand its potential in causing the development of CVD. The findings from our analysis are consistent with results reported by Avan et al., [24] and De Luca et al., [58], where they also observed an association between the hs-CRP levels and CVD-risk in PLWH on HAART. In addition, in the Strategic Management of Antiretroviral Therapy Study (SMART), it has been shown that hs-CRP levels are associated with CVD events and all-cause mortality risk [23, 62, 63]. It's essential to note that the relationship between HIV, HAART, and inflammation is complex, and response among individuals may vary due to lifestyle and geographic locations. Furthermore, different antiretroviral drugs may have varying effects on inflammation. In cases where individuals on HAART show higher CRP levels compared to those not on HAART, a thorough investigation of factors such as medication adherence and potential concurrent health conditions is essential. Also, consistent monitoring and open discussions with healthcare providers are vital for optimizing HIV management and overall well-being.
Abnormal lipid profiles including raised levels of total cholesterol, LDL-c, and glycerides, to reduced concentrations of HDL-c have become reliable markers to evaluate CVD-risk in pathological settings [27, 64]. This explains routine measurement of lipid profiles in PLWH, including those on HAART [35, 65]. Interestingly, in our systematic review, especially in the data emanating from Africa, there was a strong correlation between elevated hs-CRP levels and abnormal lipid parameters, suggesting a potential increased risk of CVD in PLWH on HAART [27, 35, 45, 46, 50, 65, 66], which occurs regardless of the perceived benefits of treatment. Furthermore, our meta-analysis showed that HDL-c levels were significantly reduced in PLWH on HAART compared to controls (Fig. 4). Whereas the levels of LDL-c, total cholesterol, and triglycerides were significantly elevated in PLWH on HAART compared to controls (Figs. 4 and 5). Thus, the altered blood lipid status, as confirmed by evidence synthesized in this study, shows that PLWH on HAART indeed have an increased risk of developing CVD. This was in accordance with previously reported studies, which indicated that PLWH on HAART have an increased risk of developing CVD [27, 45, 65]. The increased CVD risk may relate to some of the metabolic side effects, such low bone mineral density [67], that are most pronounced in patients treated with nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) [27, 35, 45, 46, 50, 68] or protease inhibitors [47]. These findings may have clinical implications if not given enough attention. These may include implications in the development of CVD-related events such as myocardial infarction, heart failure, and peripheral artery disease because elevated levels of hs-CRP are consistent or identified in all these conditions in both PLWH and the uninfected individuals [27, 35, 69]. In addition, while the specificity of hs-CRP may be influenced by extended storage or the presence of EDTA in both serum and plasma [70,71,72], we are confident that these factors did not significantly impact the overarching conclusion of the present systematic review. This confidence stems from the fact that most studies included in the review reported on the serum levels of this acute phase protein (73%).
The current systematic review and meta-analysis is not without limitations. Noteworthy, most studies were composed of relatively small sample sizes; thus, the observations made remain to be explored in future studies with larger sample sizes. Another limitation is that most studies were cross-sectional rather than longitudinal, that may provide an advantage of following up on participants. There was also a high level of statistical heterogeneity among included studies. In this study the majority of studies included in the review reported on the serum levels of this acute phase protein (73%) as compared to plasma (27%). Furthermore, not all CVD-related outcomes were reported in the current analysis. However, there are also strengths of this review, which includes it being the first systematic review and meta-analysis that provides a comprehensive overview and meta-analysis of the association between hs-CRP and PLWH on HAART. Further providing much-needed information for evidence-based health care in terms of monitoring and limiting noncommunicable disease-related complications in PLWH on HAART.
Conclusion
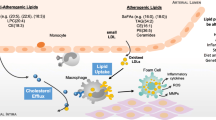
The current systematic review and meta-analysis provide evidence that elevated levels of hs-CRP and lipid profiles are prevalent in PLWH on HAART, and this may increase the risk of CVD complications (Fig. 8). These outcomes pose challenges in policy implications on care for PLWH, especially the medical care that involves the long-term use of HAART. Addressing these challenges becomes crucial for policymakers, healthcare providers, and researchers to develop strategies that balance the long-term benefits of HAART for managing HIV with the emerging concerns related to cardiovascular risks. In addition, we motivate for large sample and longitudinal studies to be undertaken to clearly elucidate the influence of HAART use on CRP levels, lipid profiles, average age groups, follow-up duration and duration of HAART treatment and its relationship with the development of CVD in PLWH.
Implication of high-sensitivity C-reactive protein (hs-CRP) and lipid profiles as a potential predictors of cardiovascular disease risk in people living with HIV (PLWH) on highly active antiretroviral therapy (HAART). HIV Human immunodeficiency virus, HDL-c non-high-density lipoprotein cholesterol, TG triglyceride, HDL-c non-high-density lipoprotein cholesterol
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- PRISMA:
-
Systematic Reviews and Meta-Analyses
- PROSPERO:
-
Prospective Register of Systematic Reviews
- SPSS:
-
Statistical Package for the Social Sciences
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- NRTIs:
-
Nucleoside/nucleotide reverse transcriptase inhibitors
- 3TC:
-
Lamivudine
- ABC:
-
Abacavir
- ATV:
-
Atazanavir
- BIC:
-
Bictegravir
- CAB:
-
Cabotegravir
- COBI or /c:
-
Cobicistat
- d4T:
-
Stavudine
- ddI:
-
Didanosine
- DMPA:
-
Depot medroxyprogesterone acetate
- DOR:
-
Doravirine
- DRV:
-
Darunavir
- DTG:
-
Dolutegravir
- EFV:
-
Efavirenz
- ETR:
-
Etravirine
- EVG:
-
Elvitegravir
- FPV:
-
Fosamprenavir
- FTC:
-
Emtricitabine
- FTR:
-
Fostemsavir
- IBA:
-
Ibalizumab
- IDV:
-
Indinavir
- LPV:
-
Lopinavir
- MVC:
-
Maraviroc
- NFV:
-
Nelfinavir
- NVP:
-
Nevirapine
- RAL:
-
Raltegravir
- RPV:
-
Rilpivirine
- RTV or /r:
-
Ritonavir
- SQV:
-
Saquinavir
- T-20:
-
Enfuvirtide
- TAF:
-
Tenofovir alafenamide
- TDF:
-
Tenofovir disoproxil fumarate
- TFV:
-
Tenofovir
- TFV-DP:
-
Tenofovir diphosphate
- Tris:
-
Tris(hydroxymethyl)aminomethane
- TMP-SMX:
-
Trimethoprim sulfamethoxazole
- TMR:
-
Temsavir
- TPV:
-
Tipranavir
- ZDV:
-
Zidovudine
- EFZ:
-
Efavirenz
- VLDL:
-
Very low-density lipoprotein
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- HDL:
-
High density lipoprotein cholesterol
- LDL:
-
Low density lipoprotein cholesterol
- hs-CRP:
-
High sensitivity C-reactive protein
- TNF-α:
-
Tumor necrosis factor
- IL-6:
-
Interleukin
- IL-8:
-
Interleukin
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular diseases
- PLWH:
-
Human immunodeficiency virus
- HAART:
-
High active antiretroviral therapy
References
World health statistics 2021: monitoring health for the SDGs, sustainable development goals. 2022.
Scheibe A, et al. Money, power and HIV: economic influences and HIV among men who have sex with men in sub-Saharan Africa. Afr J Reprod Health. 2014;18(1):84–92.
Birdthistle I, et al. Recent levels and trends in HIV incidence rates among adolescent girls and young women in ten high-prevalence African countries: a systematic review and meta-analysis. Lancet Glob Health. 2019;7(11):e1521–40.
Murray CJ, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384(9947):1005–70.
World Health Organization. World Health Statistics 2016 [OP]: Monitoring Health for the Sustainable Development Goals (SDGs). World Health Organization. 2016.
Trickey A, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. The lancet HIV. 2017;4(8):e349–56.
Feinstein MJ. HIV, Subclinical Cardiovascular Disease, and Clinical Progression: Insights From Immunologic Heterogeneity. JAMA. 2022;328(10):931–2.
Zhou B, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19· 1 million participants. The Lancet. 2017;389(10064):37–55.
Nittayananta W, et al. Effects of long-term use of HAART on oral health status of HIV-infected subjects. J Oral Pathol Med. 2010;39(5):397–406.
Lu S, Bao MY, Miao SM, Zhang X, Jia QQ, Jing SQ, et al. Prevalence of hypertension, diabetes, and dyslipidemia, and their additive effects on myocardial infarction and stroke: a cross-sectional study in Nanjing, China. Ann Transl Med. 2019;7(18):436. https://doi.org/10.21037/atm.2019.09.04.
Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–9.
Wallet MA, et al. Increased inflammation but similar physical composition and function in older-aged, HIV-1 infected subjects. BMC Immunol. 2015;16:43.
Lv T, Cao W, Li T. HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies. J Immunol Res. 2021;2021:7316456. https://doi.org/10.1155/2021/7316456.
Mahlangu T, et al. A systematic review on the functional role of Th1/Th2 cytokines in type 2 diabetes and related metabolic complications. Cytokine. 2020;126: 154892.
Nyambuya TM, et al. The Effect of Successful Antiretroviral Therapy on Immune Activation and Reconstitution in HIV Infected Adults: A Systematic Review and Meta-Analysis. AIDS Rev. 2020;23(1):1–12.
Vos AG, et al. Patterns of immune activation in HIV and non HIV subjects and its relation to cardiovascular disease risk. Front Immunol. 2021;12: 647805.
Bestawros M, Chidumayo T, Blevins M, Canipe A, Bala J, Kelly P, et al. Increased systemic inflammation is associated with cardiac and vascular dysfunction over the first 12 weeks of antiretroviral therapy among undernourished, HIV-infected adults in Southern Africa. J AIDS Clin Res. 2015;6(3):431. https://doi.org/10.4172/2155-6113.1000431.
Hackam DG, Shumak SL. C-reactive protein for the prediction of cardiovascular risk: Ready for prime-time? CMAJ. 2004;170(10):1563–5.
Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
Hsue PY, Tawakol A. Inflammation and Fibrosis in HIV: Getting to the Heart of the Matter. Circ Cardiovasc Imaging. 2016;9(3):e004427. https://doi.org/10.1161/CIRCIMAGING.116.004427.
Ahmed HA, et al. Cardiovascular risk factors and markers of myocardial injury and inflammation in people living with HIV in Nairobi, Kenya: a pilot cross-sectional study. BMJ Open. 2022;12(6): e062352.
Group, M.R., et al. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Am J Epidemiol. 1996;144(6):537–47.
Buckley DI, et al. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the US Preventive Services Task Force. Ann Intern Med. 2009;151(7):483–95.
Avan A, et al. Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J Cell Physiol. 2018;233(11):8508–25.
Fu Y, Wu Y, Liu E. C-reactive protein and cardiovascular disease: From animal studies to the clinic (Review). Exp Ther Med. 2020;20(2):1211–9.
Goedel A, et al. Influence of antiretroviral therapy and cardiovascular disease on the immature platelet fraction in patients living with HIV. Platelets. 2020;31(6):756–62.
Appiah LT, et al. Lipoprotein(a) and High Sensitivity C-Reactive Protein among Patients with HIV in Ghana: The Study on Cardiovascular Risk Profile of HIV-Infected Patients on HAART (SCRIPT). Glob Heart. 2020;15(1):74.
Survival after introduction of HAART in people with known duration of HIV-1 infection. The CASCADE Collaboration. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355(9210):1158–9.
Parums DV. Review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Medical science monitor: international medical journal of experimental and clinical research. 2021;27:e934475–81.
Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–9.
O’Connor SR, et al. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes. 2015;8:224.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clinical Epidemiology and Global Health. 2019;7(2):192–8.
Zhou DT, et al. Changes in coronary heart disease risk profiles of HIV patients in Zimbabwe over 9 months: a follow-up study. HIV AIDS (Auckl). 2016;8:165–74.
Di Yacovo, S. and M. Saumoy, Lipids, biomarkers, and subclinical atherosclerosis in treatment-naive HIV patients starting or not starting antiretroviral therapy: Comparison with a healthy control group in a 2-year prospective study. 2020. 15(8): p. e0237739.
Botha S, Fourie CM, van Rooyen JM, Kruger A, Schutte AE. Cardiometabolic changes in treated versus never treated HIV-infected black South Africans: the PURE study. Heart Lung Circ. 2014;23(2):119–26. https://doi.org/10.1016/j.hlc.2013.07.019.
Dong J, et al. The Associations of Lipid Profiles With Cardiovascular Diseases and Death in a 10-Year Prospective Cohort Study. Front Cardiovasc Med. 2021;8: 745539.
Fourie CM, et al. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis. 2015;240(1):154–60.
Borkum M, et al. High prevalence of “non-dipping” blood pressure and vascular stiffness in HIV-infected South Africans on antiretrovirals. PLoS ONE. 2017;12(9): e0185003.
Muswe R, et al. Inflammatory markers and plasma lipids in HIV patients: a correlation analysis study. The Open Biochemistry Journal. 2017;11:105.
Organization, W.H., Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach-2010 revision. 2010: World Health Organization.
Organization WH, World health statistics 2023: monitoring health for the SDGs, sustainable development goals. World Health Organization. 2023.
Mutevedzi PC, et al. Decreased chronic morbidity but elevated HIV associated cytokine levels in HIV-infected older adults receiving HIV treatment: benefit of enhanced access to care? PLoS ONE. 2013;8(10): e77379.
Canipe A, et al. A 12 week longitudinal study of microbial translocation and systemic inflammation in undernourished HIV-infected Zambians initiating antiretroviral therapy. BMC Infect Dis. 2014;14:521.
Ssinabulya I, et al. Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS ONE. 2014;9(2): e89537.
Gleason RL Jr, et al. Current Efavirenz (EFV) or ritonavir-boosted lopinavir (LPV/r) use correlates with elevate markers of atherosclerosis in HIV-infected subjects in Addis Ababa, Ethiopia. PLoS ONE. 2015;10(4): e0117125.
Borkum MS, et al. High prevalence of “non-dipping” blood pressure and vascular stiffness in HIV-infected South Africans on antiretrovirals. PLoS ONE. 2017;12(9): e0185003.
Muswe R, et al. Inflammatory Markers and Plasma Lipids in HIV Patients: A Correlation Analysis Study. Open Biochem J. 2017;11:105–18.
Hurwitz BE, et al. HIV, metabolic syndrome X, inflammation, oxidative stress, and coronary heart disease risk : role of protease inhibitor exposure. Cardiovasc Toxicol. 2004;4(3):303–16.
Boger MS, et al. Highly sensitive C-reactive protein, body mass index, and serum lipids in HIV-infected persons receiving antiretroviral therapy: a longitudinal study. J Acquir Immune Defic Syndr. 2009;52(4):480–7.
Ticona E, et al. Biomarkers of inflammation in HIV-infected Peruvian men and women before and during suppressive antiretroviral therapy. AIDS (London, England). 2015;29(13):1617.
Hileman C, et al. Relationship between total bilirubin and endothelial function, inflammation and oxidative stress in HIV-infected adults on stable antiretroviral therapy. HIV Med. 2012;13(10):609–16.
Desvarieux M, et al. Infection duration and inflammatory imbalance are associated with atherosclerotic risk in HIV-infected never-smokers independent of antiretroviral therapy. AIDS. 2013;27(16):2603–14.
Syed SS, et al. Assessment of biomarkers of cardiovascular risk among HIV type 1-infected adolescents: role of soluble vascular cell adhesion molecule as an early indicator of endothelial inflammation. AIDS Res Hum Retroviruses. 2013;29(3):493–500.
Calmy A, et al. HIV increases markers of cardiovascular risk: results from a randomized, treatment interruption trial. AIDS. 2009;23(8):929–39.
Padilla A, et al. Early changes in inflammatory and pro-thrombotic biomarkers in patients initiating antiretroviral therapy with abacavir or tenofovir. BMC Infect Dis. 2011;11(1):1–6.
Ghislain M, et al. Late Antiretroviral Therapy (ART) Initiation Is Associated with Long-Term Persistence of Systemic Inflammation and Metabolic Abnormalities. PLoS ONE. 2015;10(12): e0144317.
De Luca A, et al. The association of high-sensitivity c-reactive protein and other biomarkers with cardiovascular disease in patients treated for HIV: a nested case-control study. BMC Infect Dis. 2013;13:414.
Organization, W.H., World health statistics 2023: monitoring health for the SDGs, sustainable development goals. 2023: World Health Organization.
Peer N, Nguyen KA, Hill J, Sumner AE, Cikomola JC, Nachega JB, Kengne AP. Prevalence and influences of diabetes and prediabetes among adults living with HIV in Africa: a systematic review and meta-analysis. J Int AIDS Soc. 2023;26(3):e26059. https://doi.org/10.1002/jia2.26059.
Yuyun MF, et al. Cardiovascular Diseases in Sub-Saharan Africa Compared to High-Income Countries: An Epidemiological Perspective. Glob Heart. 2020;15(1):15.
Neuhaus J, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–95.
Hsue PY, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23(9):1059–67.
Nordestgaard BG, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–53.
Feeney ER, Mallon PW. HIV and HAART-Associated Dyslipidemia. Open Cardiovasc Med J. 2011;5:49–63.
Friis-Møller N, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study Aids. 2003;17(8):1179–93.
Holec AD, et al. Nucleotide reverse transcriptase inhibitors: a thorough review, present status and future perspective as HIV therapeutics. Curr HIV Res. 2017;15(6):411–21.
Behrens GM. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(16):1721–2 author reply 1721–2.
Duprez DA, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE. 2012;7(9): e44454.
Ishikawa S, Kayaba K, Gotoh T, Nakamura Y, Kario K, Ito Y, Kajii E. Comparison of C-reactive protein levels between serum and plasma samples on long-term frozen storage after a 13.8 year interval: the JMS Cohort Study. J Epidemiol. 2007;17(4):120–4. https://doi.org/10.2188/jea.17.120.
Nordin G, Samuelsson I, Andersson B, Börjeson J. C-reactive protein: the difference between quantitation is serum and EDTA plasma. Scand J Clin Lab Invest. 1996;56(2):123–7. https://doi.org/10.3109/00365519609088598.
Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117(2):104–11. https://doi.org/10.1016/j.clim.2005.08.004.
Acknowledgements
S.E. Mabhida and K.Z. Ziqubu were partially supported by funding from the SAMRC through its division of Research Capacity Development under the Intra-Mural Postdoctoral and doctoral Fellowship Programme from funding received from the South African Treasury. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the SAMRC or the funders.
Funding
Phiwayinkosi V. Dludla is supported in part by the National Research Foundation (NRF) (Grant numbers: 117829 and 141929). Sidney Hanser is also funded by the NRF (Grant number: TTK2204082828). The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the NRF.
Author information
Authors and Affiliations
Contributions
Authors, Sihle E. Mabhida, Haskly Mokoena, Joel Choshi, Phiwayinkosi V. Dludla, and Sidney Hanser conceived and contributed to drafting the original manuscript. Sihle E. Mabhida and Phiwayinkosi V. Dludla analysed and interpreted the data. All other authors, including Khanyisani Ziqubu, Charity Masilela, Bongani B. Nkambule, Kabelo Mokgalaboni, Zandile J.R. Mchiza, Duduzile E. Ndwandwe, and André P. Kengne reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Search strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mabhida, S.E., Mchiza, Z.J., Mokgalaboni, K. et al. High-sensitivity C-reactive protein among people living with HIV on highly active antiretroviral therapy: a systemic review and meta-analysis. BMC Infect Dis 24, 160 (2024). https://doi.org/10.1186/s12879-024-09050-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09050-4