Abstract
Background
Spontaneous miscarriage, a leading health concern globally, often occurs due to various factors, including infections. Among these, Coxiella burnetii and Brucella spp. may have adverse effects on pregnancy outcomes. While previous research has established a link between infections and spontaneous miscarriage, our study aimed specifically to investigate the presence of these two pathogens in abortion samples from women who experienced spontaneous miscarriages in Iran. Our study can add to the existing knowledge by focusing on Iran, a region with a high prevalence of C. burnetii and Brucella spp. As a result, it could provide a better understanding and unique insights into the relationship of these pathogens with spontaneous miscarriages in endemic regions.
Methods
From March 2021 to March 2022, a total of 728 abortion samples (including placenta and cotyledon) were collected from 409 women who had experienced spontaneous miscarriages in the provinces of Tehran, Fars, and West Azerbaijan in Iran. The specimens included 467 Formalin-Fixed Paraffin-Embedded (FFPE) and 261 fresh frozen samples. After DNA extraction from abortion samples, the quantitative real-time PCR (qPCR) assay targeted a specific fragment of the IS1111 and IS711 elements for molecular identification of C. burnetii and Brucella spp., respectively. Furthermore, the qPCR assay employing specific primers for different species was used to determine the species of Brucella.
Results
Among the studied women, 1 out of 409 (0.24%) samples tested positive for Brucella spp., specifically Brucella melitensis. There were no positive specimens for C. burnetii.
Conclusions
Our study contributes to understanding the potential involvement of Brucella species in spontaneous infectious abortion within endemic regions. The identification of B. melitensis in this study highlights the need for further research in this area. However, while our results suggest a relatively low or zero identification of these pathogens in our sample population, this does not rule out the possibility of undetected infections. Therefore, it is critical to acknowledge the limitations of the molecular techniques used (qPCR), which may have potential limitations such as sensitivity and specificity. Moreover, because 64.15% of our samples were FFPE, the sensitivity of the qPCR test may be reduced. These raise concerns about the accuracy of the reported prevalence rates and the potential for false positives or negatives.
Similar content being viewed by others
Background
Miscarriage is defined as the spontaneous termination of pregnancy and is estimated to occur in approximately 15–20% of pregnancies. While the exact causes of miscarriage remain unclear, several common factors contribute to this occurrence, including genetic abnormalities, uterine abnormalities, maternal age, maternal overweight, hypertension during pregnancy, hormonal imbalances, immunological factors, history of miscarriage, smoking, alcohol consumption, psychological stress, and infections [1,2,3]. 15% of early abortions and 66% of late abortions are caused by infections [1]. Among the infections, some pathogens have been implicated in increasing the risk of spontaneous miscarriages. For instance, infections caused by bacteria such as Listeria monocytogenes, Ureaplasma urealyticum, and Chlamydia trachomatis have been associated with adverse pregnancy outcomes, including miscarriages [4]. Additionally, certain viral infections like cytomegalovirus (CMV) and herpes simplex virus (HSV) have also been linked to an increased risk of miscarriages [5]. These infections can lead to inflammation, placental damage, and ultimately fetal loss [6, 7]. However, the mechanisms responsible for infectious miscarriages are diverse and can involve direct fetal infection, placental injury, and severe maternal illness. In many cases, despite the presence of elevated levels of maternal inflammatory markers or histological evidence of chorioamnionitis, no specific pathogenic agents have been identified. Intracellular bacteria, such as Coxiella burnetii and Brucella, which cannot be easily detected using conventional culture media routinely used for pathogen detection in clinical specimens, may play a significant role as etiological agents of miscarriages [1, 4]. However, their contribution is likely underestimated [1]. Advancements in modern diagnostic techniques, particularly molecular methods, have led to increased identification of infectious agents that can cause pregnancy complications, including spontaneous miscarriages [4].
Coxiella burnetii is a Gram-negative bacterium and the etiological agent of the zoonotic disease known as Q fever. The main way of contracting Q fever is through the inhalation of aerosols and particles contaminated with C. burnetii. Direct contact and the consumption of unpasteurized dairy products are less common methods of transmitting the infection to humans [8, 9]. Q fever can cause various clinical manifestations in humans, ranging from asymptomatic to acute Q fever (resembling flu-like symptoms, pneumonia, and hepatitis) and chronic Q fever (endocarditis) [10]. During pregnancy, both acute and chronic Q fever can lead to complications such as premature delivery, growth retardation, fetal abnormalities, low birth weight, stillbirth, and abortion [11]. C. burnetii not only colonizes but also multiplies in various parts of the body, including the uterus, placenta, monocytes, macrophages, and human trophoblastic cells [12]. The prevalence of C. burnetii among pregnant women in Iran has been reported to be approximately 30% [13]. Q fever is endemic in Iran, and acute Q fever and Q fever endocarditis cases have been reported in various studies [14, 15]. The incidence of Q fever during pregnancy is likely underestimated, and there is limited information available regarding the role and prevalence of this bacterium in pregnancy complications such as miscarriages. Hence, it is of utmost importance to investigate the presence of C. burnetii in miscarriages, especially considering the high seroprevalence among livestock and milk in Iran [16, 17].
Brucella is a Gram-negative bacterium that causes the disease known as brucellosis. Humans typically contract the disease through direct contact with infected animals, consuming contaminated animal products, or inhaling airborne agents [18]. There are various Brucella species, such as B. abortus, B. melitensis, B. suis, and B. canis [19], all of which can cause brucellosis in humans. This disease can lead to complications during pregnancy, including miscarriage, premature birth, contagious or neonatal brucellosis, intrauterine infection, or intrauterine fetal death [20]. Brucellosis is an endemic disease in Iran and has been reported in different regions of the country; the prevalence rate varies from 98 to 130 per 100,000 population [21, 22]. In endemic areas, the prevalence rates of brucellosis in pregnant women range from 1.3 to 12.2% [23]. A study in Iran found that 53% of pregnant women with brucellosis experienced spontaneous miscarriages [24]. In Iran, there is a lack of research on the genetic proof of Brucella in women who have had abortions, making it imperative to examine the presence of Brucella in these women.
While the role of C. burnetii and Brucella spp. in spontaneous miscarriages is well-documented, our study focuses specifically on Iran, a region with a high prevalence of these pathogens, providing unique insights into their impact on spontaneous miscarriages. Therefore, this study aimed to investigate the presence of Brucella and C. burnetii in abortion samples from women with a history of abortion in Iran, as these infectious and miscarriage abortions are prevalent health issues in the country due to the endemic nature of both Brucella and C. burnetii.
Methods
Sample collection
We conducted a completely random sampling method between March 2021 and March 2022 and collected 728 abortion tissue samples, including placenta and cotyledon, from 409 pregnant women in Tehran, West Azerbaijan, and Fars provinces (Fig. 1). The choice of these provinces was based on their geographic representation and accessibility of samples from biobanks and rural areas.
Our sample collection strategy was designed to maximize the diversity of our study population. Therefore, we collected samples from both urban and rural settings to account for differences in exposure to the pathogens of interest.
In this study, 261 fresh frozen samples were obtained from the biobanks of Urmia University of Medical Sciences (West Azerbaijan Province) and Shahid Beheshti University of Medical Sciences (Tehran Province), as well as from rural women with a history of abortion. We also included 467 formalin-fixed and paraffin-embedded (FFPE) blocks of placenta samples from rural women, which were accessible at Shiraz University of Medical Sciences in Fars Province.
Participants were selected based on having experienced a spontaneous miscarriage. They were required to provide informed consent before participation. The age of the participants ranged from 15 to 43 years. Age was chosen as a criterion because older ages have been associated with a higher risk of spontaneous miscarriages.
Sample preparation
The fresh frozen samples were prepared by cutting up to 25 mg into thin sections using a sterile razor blade.
For FFPE samples, the sections were first deparaffinized using xylene. Deparaffinization removes the paraffin wax from the tissue sections, which is necessary for subsequent DNA extraction steps. After deparaffinization, the samples were treated with ethanol solutions of varying concentrations (100, 90, 80, and 60%, respectively) for xylene elimination and tissue hydration. This step helps to remove the residual xylene from the tissue samples and to hydrate the tissue, making it easier to extract DNA. Finally, up to 25 mg of these tissues were stored for DNA extraction.
DNA extraction
DNA was extracted from the prepared tissue samples using the Animal Tissue DNA Isolation Kit (Viragen Co., Iran) according to the manufacturer’s instructions. Finally, the absorbance at 260 nm, 280 nm, and 230 nm of the extracted DNA was evaluated for quantity and quality using a NanoDrop (Epoch BioTek, USA). Absorbance ratios of 260/280 nm ~ 1.8 and 260/230 nm ~ 2.0-2.2 were considered good quality.
Identification of C. burnetii and Brucella spp.
To identify C. burnetii and Brucella spp., TaqMan quantitative real-time PCR (qPCR) was conducted on extracted DNA samples using the primers and probes that were previously published and designed [25, 26] to target highly conserved regions of the IS1111 and IS711 genes, respectively. The qPCR was carried out in a final volume of 20 µl, containing 10 µl of commercial master mix 2x Real Q Plus Master Mix for Probe (Ampliqon Co., Denmark), 900 nmol of forward primer, 900 nmol of reverse primer, 200 nmol of probe (Table 1), 4 µl (1–100 ng) of DNA template, and distilled water to reach the final volume. The amplification process was conducted in a Corbett 6000 Rotor-Gene system thermocycler (Corbett, Victoria, Australia) with the following program: initial denaturation at 95 °C for 10 min, followed by 45 cycles at 95 °C for 15 s and 60 °C for 60 s.
To ensure the reliability of our results, several validation steps were undertaken. Firstly, all qPCR assays were evaluated for their analytical sensitivity using serial dilutions of known positive DNA samples, and the lower limit of detection (LOD) was determined. Secondly, positive and negative controls were included in each qPCR run. Distilled water (no template DNA) was used as a negative control, while the C. burnetii Nine Mile RSA493 strain and a known positive DNA extracted from clinical strain for B. abortus served as positive controls for C. burnetii and Brucella spp., respectively. A sample was considered positive for a particular pathogen if it showed amplification with a quantification cycle (Cq) value below the LOD and if the amplification curve matched the expected pattern. Samples yielding Cq values above the LOD were considered negative or inconclusive.
Brucella species identification
After performing the qPCR analysis to identify the Brucella genus in the previous step, a second qPCR assay was conducted using specific primers and probes to detect the different Brucella species, such as B. melitensis and B. abortus (Table 1). The PCR conditions remained consistent with those mentioned in the previous section.
Results
Out of the 728 abortion samples, there were 50 frozen samples from Tehran Province, 211 samples from West Azerbaijan, and 476 FFPE samples from Fars Province belonging to 148 aborted women. The average (± SD) age of the women with spontaneous abortion was 29 years (range: 15–43 years).
In fresh frozen samples, 111(51.39%) were collected from urban areas, while 105 (48.61%) were from rural regions. However, all 467 FFPE samples were originated from rural locations.
Regarding the stage of pregnancy, we currently have information available only for patients with fresh frozen samples, and not for FFPE samples. In our study, the majority of spontaneous abortion cases in patients with fresh frozen samples occurred in the first trimester of pregnancy (week 1 - week 12), making up 53.95% of cases. In the second trimester (week 13 - week 28), there were 116 cases (53.95%). Interestingly, there were no cases of spontaneous abortion recorded in the third trimester (week 29 - week 40).
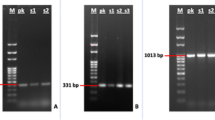
Among the 728 samples obtained from 409 women, only one sample was positive for Brucella spp. using the qPCR method. This sample had a Cq value of 28.5. In a second specific qPCR assay, the positive sample was identified as B. melitensis with a Cq value of 23.36. The sample belonged to a 23-year-old worker woman with a weight of 54 kg who experienced a spontaneous abortion on day 83 (first trimester) of her pregnancy. This woman resided in a rural area of West Azerbaijan Province and had no history of previous miscarriages or underlying diseases.
Furthermore, the present study revealed that none of the samples tested positive for C. burnetii.
Discussion
In this study, the prevalence of C. burnetii and Brucella was investigated in samples from miscarriages. Miscarriage is a common complication of pregnancy that can occur frequently or sporadically [28]. Some intracellular bacteria, including L. monocytogenes, C. trachomatis, C. burnetii, and Brucella, have been found to affect embryonic units and cause serious diseases in both the mother and fetus [4]. For instance, a study conducted in Switzerland from 2006 to 2009 revealed that the prevalence of IgG against C. trachomatis was more common in the group experiencing miscarriages (15.2%) compared to the control group (7.3%; p = 0.018). Additionally, the DNA of C. trachomatis was more frequently detected from the products of conception or placenta in women who had miscarriages (4%) compared to those in the control group (0.7%; p = 0.026) [29]. However, few studies have been conducted on Q fever infections in pregnant women and the associated abnormal and complex complications. Adverse pregnancy outcomes resulting from Q fever infections in pregnant women are typically caused by vascular thrombosis, which leads to placental insufficiency and abortion. Studies have also reported direct infection of the fetus [30]. Serological studies of C. burnetii among pregnant women living in rural areas with direct contact with livestock were found to be 29.3% in southwestern and northern Iran and 48.4% in western Iran [13, 31]. However, our study did not detect any C. burnetii infections using the molecular method.
Acute Q fever infection during the first trimester of pregnancy significantly increases the risk of both the mother and fetus developing chronic Q fever infection. Furthermore, an asymptomatic infection during pregnancy can progress to a chronic state, increasing the chances of reinfection in future pregnancies [32, 33].. In Denmark, a study found a significantly higher seroprevalence rate (47%) of Q fever among pregnant women who were exposed to livestock than among nonexposed women (4.8%) [34].
Several studies have investigated the prevalence of C. burnetii in abortion samples from pregnant women using molecular methods. One study conducted in Turkey on 51 placental samples found no detection of C. burnetii [35]. Similarly, a study in France on 246 placenta samples also reported no infection with this pathogen [36], which is consistent with our findings. However, a study in Algeria reported a positive rate of 0.55% in placental samples [37]. In Iraq, a molecular study reported that 17.02% of blood samples from aborted women were positive for C. burnetii [38]. Many studies have focused on the prevalence of Q fever in pregnant women using serological methods. In two separate studies conducted in Turkey, the seroprevalence of Q fever among pregnant women was found to be 20.7% and 14%, respectively [35, 39]. In Iran, the seroprevalence of C. burnetii infection in pregnant women was found to be 22% in the southwest (Ahvaz City) and 36.5% in the north (Parsabad City). Furthermore, the overall prevalence of C. burnetii infection in women with a history of abnormal pregnancy (39.8%) was significantly higher than that in women with normal pregnancies (23.8%) [13]. Additionally, a study conducted among rural pregnant women in Khorramabad (western Iran), who were in contact with livestock, reported a seroprevalence of 48.4% for C. burnetii [31]. However, a possible explanation for the non-detection of C. burnetii in abortion samples during this study could be due to the exclusion of specific infection foci in the examined tissue samples. In addition, it is plausible that the infection was latent or had already resolved by the time of examination, rendering the molecular test insufficient for diagnosis.
Although the presence of erythritol in the placenta of livestock acts as a growth factor for Brucella, cases of abortion have also been reported in humans [40,41,42]. The first case of brucellosis in a pregnant woman was reported in 1908 in a rural area [43]. In Egypt, the seroprevalence of this disease in pregnant women in endemic areas was found to be 12% [44].. In our study, a positive case of Brucella was only reported in the abortion sample of a pregnant woman living in a rural area. This positive sample was classified as B. melitensis, which causes brucellosis in humans and is known to primarily cause abortion and stillbirth [19]. In Saudi Arabia, a study reported a 3.5% prevalence of brucellosis in women living in rural areas. Furthermore, the incidence of abortion in pregnant women with Brucella titers greater than 1:160 was found to be 17.6%, while the incidence of abortion in women with a lower titer was significantly lower at 7.7% [45]. In 1954 in Spain, among 200 pregnant women infected with B. melitensis, 10% of these women experienced abortion in the first trimester (34). In Iran, the first study on human spontaneous abortion conducted in 1974 included 51 women from endemic regions in Isfahan Province who had abortions during their second trimester of pregnancy. Among these patients, six showed clinical and laboratory evidence of brucellosis infection, with five positive cultures of fetal remains and placenta samples [46].
In a study in Turkey, 24.1% of infected pregnant women suffered a miscarriage, whereas only 7.6% of the control group tested positive for brucellosis [47]. In Pakistan in 2016, the seroprevalence of Brucella in pregnant women was found to be 5.8%, with a significant rate of positive cases in rural areas. In addition, seroprevalence was reported to be 14.6% in women who experienced their first abortion, 12.5% in women in contact with infected animals, and 15.8% in women with a history of multiple abortions [20]. In a study in London in 2009, the seroprevalence of C. burnetii and Brucella among 438 pregnant women was found to be 4.6% and 0.5%, respectively [48]. Finally, in another study in Pakistan in 2021, it was discovered that 25.5% of women with a history of abortion were positive for Brucella using molecular methods. The predominant species identified in this study was B. abortus [49].
Since spontaneous miscarriages can be influenced by a multitude of factors beyond infections, such as B. melitensis, it should be noted that in this study and most studies, no adjustment was made for several risk factors that could be considered potential confounders. For instance, hypertensive disorders of pregnancy (HDP), which affect around 5–10% of pregnancies worldwide, can lead to complications such as fetal loss [50]. A study conducted in the Southern United States from 2000 to 2012 found that women with at least one fibroid were more likely to experience a miscarriage compared to those without fibroids. The odds ratio was 4.33 for women aged 35 years and above [51]. In addition, one study found that Polycystic ovary syndrome (PCOS), a common endocrine disorder, was present in 59.8% of women with recurrent miscarriages [52]. Our study was also limited by restricted access to patients’ medical records, a crucial limitation that impacted the comprehensive gathering and analysis of data. Consequently, multiple potential risk factors were not considered during the adjustment process.Therefore, it should be noted that other potential causes of spontaneous miscarriages, make it challenging to contextualize the significance of infection among other known risk factors.
Conclusion
In this study, we were unable to detect any positive samples for C. burnetii, although only one sample was found to be infected with B. melitensis. However, our results should not be interpreted as ruling out the possibility of miscarriage in pregnant women due to infection with these two pathogens. Acknowledging the limitations of qPCR for identification, it is important to note that the technique may have potential limitations in terms of sensitivity and specificity. False positives or negatives are possible, and further research would be valuable to validate our findings using alternative diagnostic methods. Moreover, because a significant proportion (64.15%) of our samples were derived from FFPE, it could contribute to a possible reduction in the sensitivity of the qPCR test used in this study. It is crucial to consider these factors when interpreting the results of the study. In addition, the study was conducted on a relatively small sample size. Therefore, to confirm our findings and increase statistical power, future studies should consider recruiting a larger sample size more quality and suitable samples, and additional laboratory tests. It is recommended that pregnant women residing in endemic areas for Brucella undergo screening and be monitored throughout their pregnancy using serological tests to ensure prompt identification and treatment. It is important to note that infection in pregnant women can be asymptomatic, which poses a challenge for timely diagnosis and prevention of adverse pregnancy outcomes. Consequently, healthcare systems in endemic areas should prioritize the management of these diseases.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- C. burnetii :
-
Coxiella burnetii
- qPCR:
-
quantitative real-time PCR
- B. melitensis :
-
Brucella melitensis
- B. abortus :
-
Brucella abortus
- FFPE:
-
Formalin-fixed and paraffin-embedded
- Cq:
-
Quantification cycle
References
Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22(1):116–33.
Iqbal Z, Jilanee SDA, Uppada LP, Imtiaz S, Khan H, Shah SMH, Tousif S, Rahim A, Jilanee D Jr, Uppada LJC. Evaluating the clinical risk factors Associated with miscarriages in women in Karachi. Pakistan. 2021, 13(10).
Turesheva A, Aimagambetova G, Ukybassova T, Marat A, Kanabekova P, Kaldygulova L, Amanzholkyzy A, Ryzhkova S, Nogay A. Khamidullina ZJJoCM: recurrent pregnancy loss etiology, risk factors, diagnosis, and management. Fresh look into a full box. 2023, 12(12):4074.
Baud D, Greub G. Intracellular bacteria and adverse pregnancy outcomes. Clin Microbiol Infect. 2011;17(9):1312–22.
Charostad J, Mokhtari-Azad T, Yavarian J, Ghavami N, Khorrami SMS, Behboudi E, Jalilvand S, Malekshahi SS. Shafiei-Jandaghi NZJIJoRB: detection of human herpes viruses 1–5 in miscarriage: a case-control study. 2020, 18(7):501.
Brien M-E, Baker B, Duval C, Gaudreault V, Jones RL. Girard SJCjop, pharmacology: alarmins at the maternal–fetal interface: involvement of inflammation in placental dysfunction and pregnancy complications. 2019, 97(3):206–12.
Megli CJ, Coyne CBJNRM. Infections at the maternal–fetal interface: an overview of pathogenesis and defence. 2022, 20(2):67–82.
Young EJ, Roushan MRH, Shafae S, Genta RM, Taylor SL. Liver histology of acute brucellosis caused by Brucella melitensis. Hum Pathol. 2014;45(10):2023–8.
de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella–host interactions. Am J Pathol. 2015;185(6):1505–17.
Mohabbati Mobarez A, Bagheri Amiri F, Esmaeili S. Seroprevalence of Q fever among human and animal in Iran; a systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11(4):e0005521.
Zarza SM, Mezouar S, Mege J-L. From Coxiella burnetii infection to pregnancy complications: key role of the immune response of placental cells. Pathogens. 2021;10(5):627.
Ben Amara A, Ghigo E, Le Priol Y, Lépolard C, Salcedo SP, Lemichez E, Bretelle F, Capo C, Mege J-L. Coxiella burnetii, the agent of Q fever, replicates within trophoblasts and induces a unique transcriptional response. PLoS ONE. 2010;5(12):e15315.
Khameneie MK, Asadi J, Khalili M, Abiri Z. The first serological study of Coxiella burnetii among pregnant women in Iran. Iran J Public Health. 2016;45(4):523.
Esmaeili S, Golzar F, Ayubi E, Naghili B, Mostafavi E. Acute Q fever in febrile patients in northwestern of Iran. PLoS Negl Trop Dis. 2017;11(4):e0005535.
Yaghmaie F, Esmaeili S, Francis SA, Mostafavi E. Q fever endocarditis in Iran: a case report. J Infect Public Health. 2015;8(5):498–501.
Nokhodian Z, Feizi A, Ataei B, Hoseini SG, Mostafavi E. Epidemiology of Q fever in Iran: a systematic review and meta-analysis for estimating serological and molecular prevalence. J Res Med Sciences: Official J Isfahan Univ Med Sci 2017, 22.
Esmaeili S, Mobarez AM, Khalili M, Mostafavi E. High prevalence and risk factors of Coxiella burnetii in milk of dairy animals with a history of abortion in Iran. Comp Immunol Microbiol Infect Dis. 2019;63:127–30.
Kneipp CC, Deutscher AT, Coilparampil R, Rose AM, Robson J, Malik R, Stevenson MA, Wiethoelter AK, Mor SM. Clinical investigation and management of Brucella suis seropositive dogs: a longitudinal case series. J Vet Intern Med 2023.
Lopes B, Nicolino L, PA Haddad R. J: Brucellosis-risk factors and prevalence: a review. The Open Veterinary Science Journal 2010, 4(1).
Ali S, Akhter S, Neubauer H, Scherag A, Kesselmeier M, Melzer F, Khan I, El-Adawy H, Azam A, Qadeer S. Brucellosis in pregnant women from Pakistan: an observational study. BMC Infect Dis. 2016;16:1–6.
Mirnejad R, Jazi FM, Mostafaei S, Sedighi M. Epidemiology of brucellosis in Iran: a comprehensive systematic review and meta-analysis study. Microb Pathog. 2017;109:239–47.
Aghamohammad S, Rastin M, Mostafavi E, Anaraki AH, Rahravani M, Sadaf RA, Moravedji M, Rohani M. Determination of seroprevalence of brucellosis in livestock and high-risk population in Kurdistan, Western Iran. Comp Immunol Microbiol Infect Dis. 2023;93:101942.
Bosilkovski M, Arapović J, Keramat F. Human brucellosis in pregnancy–An overview. Bosnian J Basic Med Sci. 2020;20(4):415.
Roushan MRH, Baiani M, Asnafi N, Saedi F. Outcomes of 19 pregnant women with brucellosis in Babol, northern Iran. Trans R Soc Trop Med Hyg. 2011;105(9):540–2.
Schneeberger PM, Hermans MH, van Hannen EJ, Schellekens JJ, Leenders AC, Wever PC. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin Vaccine Immunol. 2010;17(2):286–90.
Hinić V, Brodard I, Thomann A, Cvetnić Ž, Makaya P, Frey J, Abril C. Novel identification and differentiation of Brucella melitensis, B. Abortus, B. Suis, B. ovis, B. canis, and B. Neotomae suitable for both conventional and real-time PCR systems. J Microbiol Methods. 2008;75(2):375–8.
Dadar M, Alamian S, Behrozikhah AM, Yazdani F, Kalantari A, Etemadi A, Whatmore AM. Molecular identification of Brucella species and biovars associated with animal and human infection in Iran. Veterinary research forum: 2019. Urmia, Iran: Faculty of Veterinary Medicine, Urmia University; 2019. p. 315.
Rai R, Regan L. Recurrent miscarriage. The Lancet. 2006;368(9535):601–11.
Baud D, Goy G, Jaton K, Osterheld M-C, Blumer S, Borel N, Vial Y, Hohlfeld P, Pospischil A. Greub GJEid: Role of Chlamydia trachomatis in miscarriage. 2011, 17(9):1630.
Ateya HK. Molecular’’Detection of’’Coxiella Burnitii among’’Aborted women in Thi-Qar Province/Iraq Hekmat K. Ateya. Univ Thi-Qar J. 2017;12(4):1–8.
Ghobadi EA, Jaydari A, Akbari S, Anbari K. First Seroprevalence Study of Coxiella burnetii in rural pregnant women in contact with livestock in Khorramabad. Int J Infect 2019, 6(4).
Angelakis E, Million M, D’amato F, Rouli L, Richet H, Stein A, Rolain J-M, Raoult D. Q fever and pregnancy: disease, prevention, and strain specificity. Eur J Clin Microbiol Infect Dis. 2013;32:361–8.
Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A. Q fever during pregnancy: a cause of poor fetal and maternal outcome. Ann N Y Acad Sci. 2009;1166(1):79–89.
Nielsen SY, Mølbak K, Andersen AN, Henriksen TB, Kantsø B, Krogfelt K, Hjøllund N. Prevalence of Coxiella burnetii in women exposed to livestock animals, Denmark, 1996 to 2002. Eurosurveillance. 2013;18(28):20528.
Eyigör M, Gültekin B, Telli M, Odabaşı AR, Yüksel H, Aydın N. Investigation of Coxiella burnetii prevalence in women who had miscarriage and their spouses by serological and molecular methods. Mikrobiyoloji Bulteni. 2013;47(2):324–31.
Langley JM, Marrie TJ, LeBlanc JC, Almudevar A, Resch L, Raoult D. Coxiella burnetii seropositivity in parturient women is associated with adverse pregnancy outcomes. Am J Obstet Gynecol. 2003;189(1):228–32.
Ghaoui H, Bitam I, Ait-Oudhia K, Achour N, Saad-Djaballah A, Saadnia F, Kedjour S, Fournier P-E. Coxiella burnetii infection with women’s febrile spontaneous abortion reported in Algiers. New Microbes and New Infections. 2018;26:8–14.
Ateya HK. Molecular’’Detection of’’Coxiella burnitii Among’’Aborted Women in Thi-Qar Province/Iraq. Univesity of Thi-Qar Journal 2017, 12(4).
Günal Ö, Demirtürk F, Barut Ş, Kılıç S, Erkorkmaz U, Tekin F, Aysal T. A preliminary report of relationship between abortion and Q fever in Central Black Sea Region Turkish woman. Cumhuriyet Med J. 2014;36(3):337–43.
Barbier T, Machelart A, Zúñiga-Ripa A, Plovier H, Hougardy C, Lobet E, Willemart K, Muraille E, De Bolle X. Van Schaftingen EJFim: Erythritol availability in bovine, murine and human models highlights a potential role for the host aldose reductase during Brucella infection. 2017, 8:1088.
Bosilkovski M, Arapović J. Keramat FJBjobms: human brucellosis in pregnancy–An overview. 2020, 20(4):415.
Yang H-X, Feng J-J, Zhang Q-X, Hao R-E, Yao S-X, Zhao R, Piao D-R, Cui B-Y. Jiang HJIDoP: a case report of spontaneous abortion caused by Brucella melitensis biovar 3. 2018, 7(03):85–8.
Eyre J. The milroy lectures on melitensis septicæmia. The Lancet. 1908;171(4425):1747–52.
Elshamy M, Ahmed AI. The effects of maternal brucellosis on pregnancy outcome. J Infect Developing Ctries. 2008;2(03):230–4.
Sharif A, Reyes Z, Thomassen P. Screening for brucellosis in pregnant women. J Trop Med Hygiene. 1990;93(1):42–3.
Sarram i, Feiz J, Foruzandeh M, Gazanfarpour P. Intrauterine fetal infection with Brucella melitensis as a possible cause of second-trimester abortion. Am J Obstet Gynecol. 1974;119(5):657–60.
Kurdoglu M, Adali E, Kurdoglu Z, Karahocagil MK, Kolusari A, Yildizhan R, Kucukaydin Z, Sahin HG, Kamaci M, Akdeniz H. Brucellosis in pregnancy: a 6-year clinical analysis. Arch Gynecol Obstet. 2010;281:201–6.
Baud D, Peter O, Langel C, Regan L, Greub G. Seroprevalence of Coxiella burnetii and Brucella abortus among pregnant women. Clin Microbiol Infect. 2009;15(5):499–501.
Yousaf R, Khan I, Shehzad W, Hussain R, Ali S, Neubauer H, Wareth G. Seroprevalence and molecular detection of brucellosis in hospitalized patients in Lahore hospitals, Pakistan. Infect Disease Rep. 2021;13(1):166–72.
Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z. Zhang HJBp, childbirth: epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. 2021, 21(1):1–10.
Hartmann KE, Velez Edwards DR, Savitz DA, Jonsson-Funk ML, Wu P, Sundermann AC. Baird DDJAjoe: prospective cohort study of uterine fibroids and miscarriage risk. 2017, 186(10):1140–8.
Mayrhofer D, Hager M, Walch K, Ghobrial S, Rogenhofer N, Marculescu R, Seemann R, Ott JJJCM. The prevalence and impact of polycystic ovary syndrome in recurrent miscarriage: a retrospective cohort study and meta-analysis. 2020, 9(9):2700.
Acknowledgements
We would like to express our gratitude to Parisa Esmaeili for her kind help in laboratory tests.
Funding
This research was funded by the Iranian National Scientific Foundation (grant no. 91004716) and the Pasteur Institute of Iran (grant no. 2118).
Author information
Authors and Affiliations
Contributions
S.E. and E.M. helped in the conception and design of the study. A.O., N.B., and M.L. contributed to perform experiments. S.K., N.O., S.S., R.J., and S.Z. helped in sampling and facilitating the relevant procedures. A.O., N.B., M.L., A.G., and S.E. helped in data analysis. A.O. and N.B. helped in the first draft writing. N.B. revised the manuscript and responded to the reviewers’ comments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Medical Ethics Committee of the Iran Institute of Medical Sciences (NIMAD) and the National Institute of Medical Sciences Research Development of the Islamic Republic of Iran (IR.NIMAD. REC.1400.174). All methods and protocols were conducted following their guidelines and the guidelines of the Declaration of Helsinki. Written informed consent was received from every participant. In the case of participants under the age of 18, informed consent was obtained from their legal guardian(s) as well. The researchers ensured that the patients’ identities were completely hidden.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baseri, N., Omidi, A.H., Latifian, M. et al. Molecular examination for Coxiella burnetii and Brucella spp. infections in Iranian women experiencing spontaneous miscarriage. BMC Infect Dis 24, 172 (2024). https://doi.org/10.1186/s12879-024-09041-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09041-5