Abstract
Objective
This study aimed to explore the characteristics of carbapenem-resistant Enterobacterales (CRE) patients in the intensive care unit (ICU) in different regions of Henan Province to provide evidence for the targeted prevention and treatment of CRE.
Methods
This was a cross-sectional study. CRE screening was conducted in the ICUs of 78 hospitals in Henan Province, China, on March 10, 2021. The patients were divided into provincial capital hospitals and nonprovincial capital hospitals for comparative analysis.
Results
This study involved 1009 patients in total, of whom 241 were CRE-positive patients, 92 were in the provincial capital hospital and 149 were in the nonprovincial capital hospital. Provincial capital hospitals had a higher rate of CRE positivity, and there was a significant difference in the rate of CRE positivity between the two groups. The body temperature; immunosuppressed state; transfer from the ICU to other hospitals; and use of enemas, arterial catheters, carbapenems, or tigecycline at the provincial capital hospital were greater than those at the nonprovincial capital hospital (P < 0.05). However, there was no significant difference in the distribution of carbapenemase strains or enzymes between the two groups.
Conclusions
The detection rate of CRE was significantly greater in provincial capital hospitals than in nonprovincial capital hospitals. The source of the patients, invasive procedures, and use of advanced antibiotics may account for the differences. Carbapenem-resistant Klebsiella pneumoniae (CR-KPN) was the most prevalent strain. Klebsiella pneumoniae carbapenemase (KPC) was the predominant carbapenemase enzyme. The distributions of carbapenemase strains and enzymes were similar in different regions.
Similar content being viewed by others

Introduction
Carbapenem-resistant Enterobacterales (CRE) are widely disseminated in healthcare facilities worldwide and have a high mortality rate among patients due to the use of time-consuming detection methods and the limitations of treatment [1,2,3,4]. Compared to that of non-ICU isolates, the occurrence of CRE phenotypes was greater in the ICU [5]. In particular, rectal carbapenemase-producing Enterobacterales (CPE) colonization is highly prevalent in high-risk patients in the ICU [6]. Therefore, the timely and accurate detection of CRE, especially CPE, is crucial for infection prevention and clinical management, and early screening is the cornerstone of early detection. For CRE control, early detection of CRE acquisition with active surveillance may be useful [7]. Early detection and screening of CRE in patients in the ICU may promote early warning and reduce the risk of nosocomial CRE infections [8]. The use of admission screening for CRE and cohort care can significantly reduce the prevalence of CRE, the number of cases of hospital-acquired infections, and the length of hospitalization [9]. A study showed that 36.09% of CRE colonizations converted to infections. Therefore, CRE screening should be carried out in inpatients as early as possible, and effective intervention measures should be taken in a timely manner to avoid adverse consequences [10].
Pathogenic bacteria were distributed differently among different hospital departments and sample sources [11]. Thus, it is essential to target the prevention and control of key pathogenic bacteria in different hospital departments [11, 12]. However, the prevalence of CRE in different regions of Henan Province has not been reported. Zhengzhou is the most medically developed city in Henan Province, and the epidemiological characteristics of CRE may be distinctive. Therefore, to provide evidence for the targeted prevention and treatment of CRE, we screened 78 hospitals in different municipalities to determine the epidemiological characteristics of CRE in provincial and nonprovincial hospitals.
The population of Henan Province is nearly 100 million. CRE has been particularly prevalent in this area. To prevent and treat CRE in Henan Province, it is crucial to understand the characteristics of CRE in different regions.
Materials and methods
Research subjects
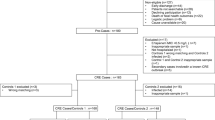
In this study, 1048 patients were enrolled from 96 ICUs in 78 hospitals in 18 cities. Patients aged older than 18 years were included. Patients with incomplete clinical data were excluded. All patients signed an informed consent form.
Of the 78 hospitals, 19 are provincial capital hospitals, all of which are tertiary hospitals, and 59 are nonprovincial capital hospitals, including 39 tertiary hospitals and 20 secondary hospitals (Table 1).
Study design and specimen collection
The trained ICU nurses collected one pharyngeal and two anal swabs per research subject on March 10, 2021. Microorganisms tested for one pharyngeal and anal swab specimen. For patients with CRE positivity, carbapenemase type was tested for in the other anal swab specimens. Following the guidelines of the People’s Republic of China Health Industry Standard and Specimen Collection and Transport in Clinical Microbiology (WS/T640-2018), samples were collected and transported. To derive samples, finish case reports, and follow up with patients, a doctor, and a nurse were assigned to each ICU.
The study was registered at http://www.chictr.org.cn/(registration number: ChiCTR2100044002, date of first registration: 06/03/2021) before patient enrollment.
Bacterial isolation and identification
Isolation, culture and identification of bacteria were performed as previously reported [13].
Identification of carbapenemase types
The most common carbapenemase types tested in all the CRE anal swabs included Klebsiella pneumoniae carbapenemase (KPC), New Delhi metallo-beta-lactamase (NDM), and oxacillinase-48 (OXA-48). The test was carried out as previously reported [13].
Statistical analysis
KolmogorovThe Smirnov test was used to estimate the distribution of continuous variables. Normally distributed data were expressed as the means ± standard deviations and compared using t tests (P < 0.05). Nonnormally distributed data are expressed as medians (interquartile ranges) and were compared using the Mann–Whitney test (P < 0.05). Categorical variables are described as proportions and were compared using the chi-square test or Fisher’s test (P < 0.05). SPSS 26.0 was used for the statistical analysis, and a P value of < 0.05 was used to indicate statistical significance. The missing data were handled by multiple imputations [14] before analysis.
Results
Basic patient information
A total of 1009 patients were included in the study. Finally, 241 patients with positive CRE detection data were included in the analysis. The patients were divided into a provincial capital hospital group and a nonprovincial capital hospital group. There was no significant difference in age, sex ratio, or BMI between the two groups. See Table 2 for details.
CRE detection rate
The CRE detection rate was 23.89% in 96 ICUs of 78 hospitals in 18 cities, 28.22% in the provincial capital hospital and 21.82% in the nonprovincial capital hospital. There was a statistically significant difference between the groups (P = 0.027). See Table 3 for details.
Laboratory tests and signs
The average body temperature was 37 °C in the Provincial Capital Hospital, which was significantly greater than that in the Nonprovincial Capital Hospital (p = 0.048). The average concentration of procalcitonin was 0.78, which was significantly greater than that in nonprovincial capital hospitals (p = 0.036). However, there were no significant differences in PaO2/FiO2, Acute Physiological and Chronic Health Evaluation Index II (APACHE II) score, white blood cell (WBC) count, C-reactive protein (CRP), creatinine, albumin, or total bilirubin. See Table 4 for details.
History of disease
Moreover, there was no statistically significant difference in the proportion of patients with comorbid diseases between the two groups. See Table 5 for details.
Patient sources
The proportion of patients transferred from ICUs to other hospitals in provincial capital hospitals was higher than that from nonprovincial capital hospitals. The proportion of patients who were newly admitted and transferred from other departments at the same hospital was greater than that from provincial capital hospitals. There was a statistically significant difference in the source of patients between the two groups (p < 0.001). See Table 5 for details.
Intrusive or invasive procedures
The proportion of patients who underwent gastroscopy, enema or arterial catheterization at the provincial capital hospital was greater than that at the nonprovincial capital. However, there were more patients who received invasive ventilation at the nonprovincial capital hospital than at the provincial capital hospital. See Table 5 for details.
History of antibiotic exposure
The proportion of patients using carbapenems and tigecycline in provincial capital hospitals was significantly greater than that in nonprovincial capital hospitals (p < 0.05). There was no significant difference in the use of other antibiotics between the two groups. See Table 5 for details.
Patients’ length of hospital stay
The average length of hospital stay was 13.00 (6.00, 26.25) days for patients in provincial capital hospitals and 12.00 (6.00, 20.00) days for patients in nonprovincial capital hospitals. The average length of ICU stay was 10.50 (5.00, 23.00) days and 10.00 (5.00, 15.00) days in the two groups, respectively. There was no statistically significant difference in the length of hospital stay or ICU stay between the two groups. See Table 6 for details.
Distribution of CRE strains
The study detected 346 cases of CRE in total, including 297 carbapenem-resistant Klebsiella pneumoniae (CR-KPN), 22 carbapenem-resistant Escherichia coli (CR-ECO), 6 carbapenem-resistant Enterobacter cloacae (CR-ECL), 19 CR-KPN/CR-ECO and 2 CR-KPN/CR-ECL strains. The isolation of CRE strains in the provincial capital hospital and in the nonprovincial capital hospital is shown in detail in Table 7. There was no statistically significant difference in the number of CRE strains detected between the two groups.
Distribution of carbapenemase enzymes
Three carbapenemase enzymes were tested in 193 positive anal swabs, 150 KPC, 9 NDM, 11 KPC, and NDM, and 23 samples failed to contain the carbapenemase enzyme tested. The detection of carbapenemase enzymes in different regional hospitals was performed as follows (Table 8), and no significant differences were found between the two groups.
Discussion
This was a multicenter cross-sectional study conducted in 96 ICUs of 78 hospitals in 18 cities; these included 19 provincial capital hospitals and 59 nonprovincial capital hospitals. Finally, 241 patients were analysed for differences in the clinical characteristics of CRE-positivity in different regions. The results revealed that the detection rate of CRE was 23.89%, 346 CRE-positive swabs were obtained, the proportion of CR-KPN was 85.84%, 193 anal swabs were tested for carbapenemase enzymes, and the proportion of KPC was 77.72%. The source of patients, invasive procedures, and history of antibiotic exposure may account for the higher detection rate of CRE in provincial capital hospitals.
Previous studies have shown that 22% of inpatients in the ICU had a subsequent CRE-positive swab [15], which is lower than the percentage in our study. The detection rate of CRE in hospitals in different regions varies. Our study showed that the detection rate of CRE was 28.22% in provincial capital hospitals and 21.82% in nonprovincial capital hospitals. According to our study, the high detection rate of CRE may be due to the source of the patients, invasive procedures, and history of antibiotic exposure. In this study, the proportion of patients transferred from ICUs to other hospitals in provincial capital hospitals was greater than that from nonprovincial capital hospitals. The patients were in critical condition and could not be handled by the local hospital. Previous studies have shown that the highest CRE positivity rate is observed in patients transferred from outside facilities to the ICU [16]. Our study showed that the proportion of patients who underwent gastroscopy, enema, or arterial catheter placement at the provincial capital hospital was greater than that at the nonprovincial capital. Interestingly, the number of patients who received invasive ventilation in nonprovincial capital hospitals was greater than that in the other hospitals. The proportion of patients using carbapenems and tigecycline in provincial capital hospitals was significantly greater than that in nonprovincial capital hospitals. Thus, CRE screening should be carried out in patients who are transferred from the ICUs of other hospitals, who are receiving invasive procedures and who are receiving advanced antibiotics as early as possible. In addition, standardized antibiotic use measures should be taken in a timely manner to avoid adverse consequences. It is effective to manage patients with CRE and non-CRE patients separately by isolating inpatients with CRE and dividing the population by the number of ICU staff [17]. Addressing sink traps can effectively reduce carbapenemase-producing Enterobacterales (CPE) transmission from patient to patient [18].
A previous study showed that a higher Sequential Organ Failure Assessment (SOFA) score, a nutritional risk in critically ill (NUTRIC) score, a prolonged intensive medical unit (ICU) length of stay (LOS), previous surgery, dialysis, mechanical ventilation during the ICU stay, and previous use of aminoglycoside and carbapenems were risk factors independently associated with CRE infection [14]. Gao et al. demonstrated that kidney disease, granulocytosis, invasive procedures, and CRE detection time were risk factors for CRE infection [10]. The invasive group had a greater rate of CRE infection than the noninvasive group [10]. Another study showed that urinary system disease, bronchoscopy, and combined antibiotic use were found to be independent risk factors for CPE colonization [6]. Otherwise, emergency department stays before ICU admission were associated with CRE colonization at admission to the ICU [19]. The length of hospital stay, hospital-acquired infection and treatment with carbapenem were found to be independent risk factors for CRE colonization [20]. A longer hospital stay was associated with an increased risk of CRE infection [21].
The study detected 346 cases of CRE in total, of which CR-KPN (85.84%) was the most prevalent strain. KPC (77.32%) is the major carbapenemase enzyme, and the detection rates of KPC and NDM were similar between the two groups. In addition, strains in which two carbapenemase enzymes were detected (KPC and NDM) were more prevalent in the provincial capital hospital. However, no significant differences were found between the two groups.
In a study by Han et al., 935 CRE strains were collected from 36 hospitals in 24 provinces or cities across China from 2016 to 2018. Overall, carbapenemases, including KPC-2 (51.6%), NDM (35.7%), and OXA-48-like carbapenemases (7.3%), were produced in 97.4% of the CRE strains [22].
Lin’s study collected 128 unique CRE isolates from 128 patients in the ICU of Chongqing, China: 69 (53.9%) CPE patients and 59 (46.1%) non-CPE patients. The most prevalent CPE isolates were bla (KPC-2) (56.5%), bla (NDM) (39.1%), and bla (IMP) (5.8%) [23].
Patients with CRE that produce carbapenemase were more likely to develop CRE infections than were those with non-carbapenemase-producing CRE [15]. KPC-CRE-colonized patients were particularly susceptible to subsequent CRE infections during their hospital stay [24]. Therefore, special attention should be given to patients with CREs harboring KPC enzymes.
Our study preliminarily confirmed the epidemiological characteristics of CRE at different hospital levels and provided a reference for the treatment of CRE locally. The limitations of our study include the small sample size and cross-sectional nature. This study did not explore the proportion of colonized CRE strains that converted to infection or distinguish CRE colonization from infection. Moreover, only common carbapenemase enzymes were tested for analysis due to the limitations of the finances. We will expand the sample size and optimize the experimental design to further validate our findings in the future.
Conclusions
The detection rate of CRE was significantly greater in the provincial capital hospitals than in the nonprovincial capital hospitals. The source of the patients, invasive procedures, and the use of advanced antibiotics may account for the differences. CR-KPN was the most prevalent strain. KPC was the predominant carbapenemase enzyme. The distributions of carbapenemase strains and enzymes were similar in different regions.
Availability of data and materials
The datasets generated from the current study are available from the corresponding author upon reasonable request.
References
Akova M, Daikos GL, Tzouvelekis L, Carmeli Y. Interventional strategies and current clinical experience with carbapenemase-producing gram-negative bacteria. Clin Microbiol Infect. 2012;18(5):439–48. https://doi.org/10.1111/j.1469-0691.2012.03823.x.
Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–8. https://doi.org/10.1016/S1473-3099(15)00424-7.
Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. Infections caused by Carbapenem-Resistant Enterobacterales: an update on therapeutic options. Front Microbiol. 2019;10:80. https://doi.org/10.3389/fmicb.2019.00080.
Bowers DR, Huang V. Emerging issues and treatment strategies in carbapenem-resistant enterobacterales (CRE). Curr Infect Dis Rep. 2016;18(12):48. https://doi.org/10.1007/s11908-016-0548-3.
Sader HS, Mendes RE, Streit JM, Carvalhaes CG, Castanheira M. Antimicrobial susceptibility of gram-negative bacteria from intensive care unit and non-intensive care unit patients from United States hospitals (2018–2020). Diagn Microbiol Infect Dis. 2022;102(1):115557. https://doi.org/10.1016/j.diagmicrobio.2021.115557.
Yan L, Sun J, Xu X, Huang S. Epidemiology and risk factors of rectal colonization of carbapenemase-producing enterobacterales among high-risk patients from ICU and HSCT wards in a university hospital. Antimicrob Resist Infect Control. 2020;9(1):155. https://doi.org/10.1186/s13756-020-00816-4.
Kang JS, Yi J, Ko MK, Lee SO, Lee JE, Kim KH. Prevalence and risk factors of carbapenem-resistant enterobacterales acquisition in an emergency intensive care unit in a tertiary hospital in Korea: a case-control study. J Korean Med Sci. 2019;34(18):e140. https://doi.org/10.3346/jkms.2019.34.e140.
Wang Y, Lin Q, Chen Z, et al. Construction of a risk prediction model for subsequent bloodstream infection in intestinal carriers of carbapenem-resistant enterobacterales: a retrospective study in Hematology Department and Intensive Care Unit. Infect Drug Resist. 2021;14:815–24. https://doi.org/10.2147/IDR.S286401.
Garpvall K, Duong V, Linnros S, et al. Admission screening and cohort care decrease carbapenem resistant enterobacterales in Vietnamese pediatric ICU’s. Antimicrob Resist Infect Control. 2021;10(1):128. https://doi.org/10.1186/s13756-021-00994-9.
Gao Y, Chen M, Cai M, et al. An analysis of risk factors for carbapenem-resistant enterobacterales infection. J Glob Antimicrob Resist. 2022;30:191–8. https://doi.org/10.1016/j.jgar.2022.04.005.
Liu G, Qin M. Analysis of the distribution and antibiotic resistance of pathogens causing infections in hospitals from 2017 to 2019. Evid Based Complement Alternat Med. 2022;2022:3512582. https://doi.org/10.1155/2022/3512582.
Chen J, Xiang Q, Wu JY, et al. Different effects of antibiotics on Klebsiella pneumoniae and Escherichia coli resistance induced by antibiotics: a retrospective study from China. Microb Drug Resist. 2022;28(6):660–9. https://doi.org/10.1089/mdr.2021.0326.
Guo B, Guo Z, Zhang H, et al. Prevalence and risk factors of carbapenem-resistant Enterobacterales positivity by active screening in intensive care units in the Henan Province of China: a multi-center cross-sectional study. Front Microbiol. 2022;13:894341. https://doi.org/10.3389/fmicb.2022.894341.
Aleidan F, Alkhelaifi H, Alsenaid A, et al. Incidence and risk factors for carbapenem-resistant enterobacterales infection in intensive care units: a matched case–control study. Expert Rev Anti Infect Ther. 2021;19(3):393–8. https://doi.org/10.1080/14787210.2020.1822736.
Tamma PD, Kazmi A, Bergman Y, et al. The likelihood of developing a carbapenem-resistant enterobacterales infection during a hospital stay. Antimicrob Agents Chemother. 2019;63(8). https://doi.org/10.1128/AAC.00757-19.
Shimasaki T, Segreti J, Tomich A, Kim J, Hayden MK, Lin MY. Active screening and interfacility communication of carbapenem-resistant Enterobacterales (CRE) in a tertiary-care hospital. Infect Control Hosp Epidemiol. 2018;39(9):1058–62. https://doi.org/10.1017/ice.2018.150.
Sakai Y, Gotoh K, Nakano R, et al. Infection control for a carbapenem-resistant enterobacterales outbreak in an advanced emergency medical services center. Antibiot (Basel). 2021;10(12):1537. https://doi.org/10.3390/antibiotics10121537.
Regev-Yochay G, Smollan G, Tal I, et al. Sink traps as the source of transmission of OXA-48-producing Serratia marcescens in an intensive care unit. Infect Control Hosp Epidemiol. 2018;39(11):1307–15. https://doi.org/10.1017/ice.2018.235.
Salomão MC, Freire MP, Boszczowski I, Raymundo SF, Guedes AR, Levin AS. Increased risk for carbapenem-resistant enterobacterales colonization in intensive care units after hospitalization in Emergency Department. Emerg Infect Dis. 2020;26(6):1156–63. https://doi.org/10.3201/eid2606.190965.
Tran DM, Larsson M, Olson L, et al. High prevalence of colonization with carbapenem-resistant Enterobacterales among patients admitted to Vietnamese hospitals: risk factors and burden of disease. J Infect. 2019;79(2):115–22. https://doi.org/10.1016/j.jinf.2019.05.013.
Zaha DC, Kiss R, Hegedűs C, et al. Recent advances in Investigation, prevention, and management of healthcare-associated infections (HAIs): resistant multidrug strain colonization and its risk factors in an intensive care unit of a University Hospital. Biomed Res Int. 2019;2019:2510875. https://doi.org/10.1155/2019/2510875.
Han R, Shi Q, Wu S, et al. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant enterobacterales isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. https://doi.org/10.3389/fcimb.2020.00314.
Lin Q, Wu M, Yu H, et al. Clinical and microbiological characterization of carbapenem-resistant enterobacteriales: a prospective cohort study. Front Pharmacol. 2021;12:716324.
Lin Q, Wang Y, Yu J, et al. Bacterial characteristics of carbapenem-resistant Enterobacterales (CRE) colonized strains and their correlation with subsequent infection. BMC Infect Dis. 2021;21(1):638. https://doi.org/10.1186/s12879-021-06315-0.
Acknowledgements
Anal and pharyngeal swab samples were provided by the following hospitals: Fuwai Huazhong Cardiovascular Hospital, the First Affiliated Hospital of Henan University, Huaihe Hospital of Henan University, First Affiliated Hospital of Henan University of Science and Technology, Yellow River Central Hospital, Henan Provincial People’s Hospital, Henan Provincial Chest Hospital, Henan NO.3 Provincial People’s Hospital, Henan Provincial Staff Hospital, Henan Provincial Hospital of Traditional Chinese Medicine, Henan Cancer Hospital, the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, the Third Affiliated Hospital of Xinxiang Medical College, the Second Affiliated Hospital of Zhengzhou University, the Fifth Affiliated Hospital of Zhengzhou University, the First Affiliated Hospital of Zhengzhou University, Hebi People’s Hospital, Yellow River Sanmenxia Hospital, Jiyuan People’s Hospital, Jiaozuo Second People’s Hospital, Jiaozuo People’s Hospital, Kaifeng People’s Hospital, Kaifeng Central Hospital, Kaifeng Traditional Chinese Medicine Hospital, Luoyang Dong Fang Hospital, Luoyang Central Hospital, Luohe Central Hospital, the Second Affiliated Hospital of Luohe Medical College, Nanshi Hospital of Nanyang, Nanyang First People’s Hospital, Nanyang Central Hospital, Pingdingshan Second People’s Hospital, Pingdingshan First People’s Hospital , Pingmei Shenma Medical Group General Hospital, Anyang District Hospital of Puyang City, Puyang People’s Hospital,Puyang Oilfield General Hospital, Puyang Hospital of Traditional Chinese Medicine, Sanmenxia Central Hospital, Shangqiu First People’s Hospital, Xinxiang Second People’s Hospital, Xinxiang First People’s Hospital, Xinxiang Central Hospital, Xinyang Central Hospital, Xuchang Municipal Hospital, Xuchang People’s Hospital, Xuchang Central Hospital, Zhengzhou People’s Hospital, Zhengzhou Sixth People’s Hospital, Zhengzhou Seventh People’s Hospital, Zhengzhou City Third People’s Hospital, Zhengzhou First People’s Hospital, Zhengzhou Orthopedic Hospital, Zhengzhou Central Hospital, Zhoukou Central Hospital, Zhumadian First People’s Hospital, Zhumadian Central Hospital, Xiangcheng County People’s Hospital, Yongcheng People’s Hospital , Zhoukou First People’s Hospital, Xinzheng People’s Hospital, Jiaxian Traditional Chinese Medicine Hospital, Xinxiang County People’s Hospital, Changyuan People’s Hospital, Bo’ai County People’s Hospital, Yexian People’s Hospital, Yanling County People’s Hospital, Yuzhou People’s Hospital, General Hospital of Yima Coal Industry Group Co., Ltd., Minquan County People’s Hospital, Xincai County People’s Hospital, Gushi County People’s Hospital, Gongyi People’s Hospital, Xinmi Traditional Chinese Medicine Hospital, Ruzhou People’s Hospital, Neihuang County People’s Hospital, Xiuwu County People’s Hospital and Dengfeng People’s Hospital.
We thank Liu Yanhong from Henan People’s Hospital for her help in the statistical analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Shao Huanzhang, Qin Bingyu, and Li Peili designed the study. Wang Shanmei conducted the carbapenem-resistant Enterobacterales detection. Guo Bo performed the statistical analyses and wrote the manuscript. The remaining authors participated in the collection of samples. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the medical ethics committee of Henan People’s Hospital (ethics number: (2020) Ethical Review No. (143)). All patients signed an informed consent form. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, B., Li, P., Qin, B. et al. An analysis of differences in Carbapenem-resistant Enterobacterales in different regions: a multicenter cross-sectional study. BMC Infect Dis 24, 116 (2024). https://doi.org/10.1186/s12879-024-09005-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09005-9