Abstract
Background
Azvudine has clinical benefits and acceptable safety against COVID-19, including in patients with comorbidities, but there is a lack of available data for its use in older adult patients. This study explored the effectiveness and safety of azvudine in older adults with mild or moderate COVID-19.
Methods
This retrospective cohort study included patients aged ≥80 diagnosed with COVID-19 at the Central Hospital of Shaoyang between October and November 2022. According to the therapies they received, the eligible patients were divided into the azvudine, nirmatrelvir/ritonavir, and standard-of-care (SOC) groups. The outcomes were the proportion of patients progressing to severe COVID-19, time to nucleic acid negative conversion (NANC), and the 5-, 7-, 10-, and 14-day NANC rates from admission.
Results
The study included 55 patients treated with azvudine (n = 14), nirmatrelvir/ritonavir (n = 18), and SOC (n = 23). The median time from symptom onset to NANC of the azvudine, nirmatrelvir/ritonavir, and SOC groups was 14 (range, 6–25), 15 (range, 11–24), and 19 (range, 18–23) days, respectively. The median time from treatment initiation to NANC of the azvudine and nirmatrelvir/ritonavir groups was 8 (range, 4–20) and 9 (range, 5–16) days, respectively. The median length of hospital stay in the three groups was 10.5 (range, 5–23), 13.5 (range, 10–21), and 17 (range, 10–23) days, respectively. No treatment-related adverse events or serious adverse events were reported.
Conclusion
Azvudine showed satisfactory effectiveness and acceptable safety in older adults with mild or moderate COVID-19. Therefore, azvudine could be a treatment option for this special patient population.
Similar content being viewed by others
Background
COVID-19 is an acute respiratory disease caused by SARS-CoV-2, a novel coronavirus closely related to SARS-CoV. The global COVID-19 pandemic has infected > 766 million people (confirmed cases) and killed > 6.9 million individuals as of May 14, 2023 [1,2,3]. The strains of SARS-CoV-2 are continuously evolving and show trends toward weaker virulence but higher infectiousness [4]. For example, the proportion of the BQ and XBB subvariants of the Omicron strain is increasing, and they manifest immune evasion [5]. The complications of COVID-19 contributing to mortality include coagulopathy, neurologic complications, and multisystem inflammatory syndrome [6, 7]. Advanced age is a high-risk factor of poor prognosis and death from COVID-19 [8, 9]. Several comorbidities known to worsen the prognosis of COVID-19 are commonly found in older adults, including cancer, cardiovascular diseases, kidney diseases, liver diseases, pulmonary conditions, rheumatic diseases, immune disorders, and musculoskeletal diseases [10,11,12,13]. The burden of disease in older adults is significant, with multiple comorbidities, severe physical or psychological symptoms, exacerbation of ageism, poor quality of life, difficult access to services, and reduced physical function [14, 15].
Antiviral drugs are considered the most effective therapies for COVID-19 and are recommended by the World Health Organization guidelines [6, 16]. The nirmatrelvir/ritonavir combination (co-packaged oral tablets, Paxlovid®) was approved for the treatment of mild-to-moderate COVID-19 in patients ≥12 years old and ≥ 40 kg with positive SARS-CoV-2 diagnostic test and who are at high risk of developing severe illness, including hospitalization or death [17, 18]. Unfortunately, antiviral drugs are expensive and in short supply, making them inaccessible to patients in developing countries. Furthermore, the treatment options for older adults with COVID-19 are limited because antiviral drugs can interact with various comorbidities to increase the risk of adverse events and may lead to nephrotoxic adverse reactions in patients with decreased kidney function or chronic kidney diseases [19, 20].
Azvudine is the first double-target nucleoside drug developed in China and was approved for COVID-19 treatment. Azvudine inhibits the nucleoside reverse transcriptase and restores cytosine deaminase APOBEC3G expression [21]. Azvudine can improve the lung function of patients with mild or moderate COVID-19, maintain their vital signs, reduce treatment time, and accelerate virus elimination [22, 23]. According to the available evidence on COVID-19 antiviral drugs, azvudine could be a therapeutic option for COVID-19 patients with comorbidities, considering its clinical benefits and acceptable safety [24,25,26]. Nevertheless, the available data on the use of azvudine in older adults are limited.
Therefore, this study aimed to explore the effectiveness and safety of azvudine in older adults with mild or moderate COVID-19. The results could help the management of COVID-19 in the population of patients with an advanced age who display multiple comorbidities and a higher risk of poor COVID-19 outcomes.
Methods
Study design and patients
This retrospective cohort study included older adult patients diagnosed with mild or moderate COVID-19 infection at the Central Hospital of Shaoyang (Hunan Province, China) between October and November 2022. This study was approved by the Ethics Committee of the Central Hospital of Shaoyang (approval number: KY-2022-002-20). Informed consent was waived by the Ethics Committee of the Central Hospital of Shaoyang due to the retrospective study nature.
The inclusion criteria were 1) ≥80 years of age, 2) positive SARS-CoV-2 (cycle threshold [Ct] value < 35) tested by reverse transcription-polymerase chain reaction (RT-PCR), 3) diagnosed with mild or moderate COVID-19 virus infection according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 9), and 4) received antiviral therapy with azvudine or nirmatrelvir/ritonavir, or only received standard-of-care (SOC) therapy. The exclusion criteria were 1) treatment started at ≥5 days after the onset of symptoms, 2) use of other antiviral agents, or 3) incomplete key clinical data, such as the nucleic acid negative conversion (NANC) time.
Grouping and treatment
According to the therapies they received, the eligible patients were divided into the azvudine, nirmatrelvir/ritonavir, and SOC groups. The dosage of azvudine was 5 mg orally once daily (QD). The dosage of nirmatrelvir/ritonavir was 300 mg of nirmatrelvir in combination with 100 mg of ritonavir administered orally twice daily (BID). Azvudine and nirmatrelvir/ritonavir were given for 5 consecutive days, and the dosages could be adjusted according to the creatinine clearance rate if necessary. The SOC therapy included traditional Chinese medicine (TCM), Lianhua Qingwen granules (LHQW), and ibuprofen. Oxygen therapy was used when the patients suffered from hypoxia.
Data collection and outcomes
Age, sex, COVID-19 severity, doses of COVID-19 vaccines, comorbidities (including cardiovascular and cerebrovascular diseases, chronic lung diseases, diabetes, chronic liver and kidney diseases, tumors, acquired immunodeficiency syndrome, immune diseases related to long-term use of glucocorticoid and immunosuppressive drugs, etc.), COVID-19 symptoms (fever, asthenia, diarrhea, cough, nasal obstruction/running nose, rigor, panting, abdominal pain, diarrhea, headache, dizziness, palpitation, chest distress, chest pain, dry/sore/itchy throat, sore muscle, nausea, vomiting, dyspnea, gasteremphraxis, gastralgia, hypogeusia/poor appetite, hyposmia, etc.), and the Ct values of COVID-19 nucleic acids were collected from the medical records. According to “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 9)”, COVID-19 was classified as mild and common types. The common type is equivalent to the European Standard for moderate COVID-19.
The outcomes were the percentage of patients with COVID-19 progressing to severe diseases, NANC times, 5-, 7-, 10-, and 14-day NANC rates from admission, and length of hospital stay (LOS). The NANC times included 1) the time from the first occurrence of symptoms to the first NANC, 2) the time from the first positive nucleic acids to the first NANC, and 3) the time from starting using antiviral drugs to the first NANC (not available in the SOC group).
The Ct values of SARS-CoV-2 nucleic acids in nasopharyngeal swabs were tested daily by RT-PCR. Ct values > 35 were considered negative. Patients were discharged after two continuous negative nucleic acid test results performed at an interval of > 24 h. Treatment-related adverse events (TRAEs) and serious adverse events (SAEs) during hospitalization were recorded, if any.
Statistical analysis
The statistical analysis was performed using R 4.2.1 (The R Project for Statistical Computing, www.r-project.org). Only descriptive statistics were used in this study. Age was presented as mean ± standard deviation, and the number of symptoms, Ct values, and NANC time were presented as medians (ranges). The categorical data were presented as n (%). The Kaplan-Meier method was used to estimate the NANC times and LOS, and the medians and 95% confidence intervals (CIs) were calculated.
Results
Characteristics of the patients
A total of 55 patients were included: 14 were treated with azvudine, 18 with nirmatrelvir/ritonavir, and 23 with SOC. In the azvudine and nirmatrelvir/ritonavir groups, one (7.1%) and three (16.7%) patients had mild COVID-19, while 21 (91.3%) had mild COVID-19 in the SOC group. The other baseline characteristics of the patients were generally balanced among the three groups (Table 1).
Clinical outcomes
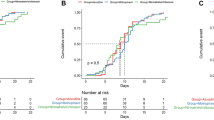
The median time from symptom onset to NANC of the azvudine, nirmatrelvir/ritonavir, and SOC groups was 14 (range, 6–25), 15 (range, 11–24), and 19 (range, 18–23) days, respectively. The median time from drug administration to NANC of the azvudine and nirmatrelvir/ritonavir groups was 8 (range, 4–20) and 9 (range, 5–16) days, respectively. The median LOS of the three groups was 10.5 (range, 5–23), 13.5 (range, 10–21), and 17 (range, 10–23) days, respectively (Fig. 1 and Table 2).
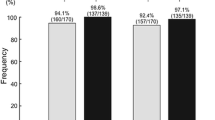
None of the patients on azvudine or nirmatrelvir/ritonavir progressed to severe COVID-19, while two (8.7%) in the SOC group progressed. The first NANC rates of the azvudine group within 5, 7, 10, and 14 days after hospital admission were 14.3, 21.4, 57.1, and 71.4%, respectively; those rates were 0, 0, 27.8, and 83.3%, respectively, in the nirmatrelvir/ritonavir group, and 0, 0, 17.4, and 43.5%, respectively, in the SOC group (Table 2).
The laboratory tests at admission and before discharge showed no clinically significant changes in liver and kidney functions in any patient. No TRAEs or SAEs were reported.
Discussion
This study showed that azvudine could shorten the NANC time compared with standard symptomatic treatment in older adults with mild or moderate COVID-19. No patient receiving azvudine progressed to severe COVID-19. No TRAEs or SAEs during hospitalization were reported.
vaccines play a major role in the control of the COVID-19 pandemic [27, 28]. Still, immune drift and mutations are observed, and the new SARS-CoV-2 variants display immune evasion to the pre-existing immunity induced by vaccines or previous infections with other SARS-CoV-2 variants [29,30,31,32]. Vaccines can only be designed based on existing SARS-CoV-2 variants, and the next variants cannot be predicted with enough precision to design vaccines before their appearance [30], but immune imprinting will provide some protection against novel variants [33]. Therefore, vaccines will always lag compared with the circulating SARS-CoV-2 variants, and antiviral drugs still have their place in the armamentarium against COVID-19.
Azvudine is a nucleoside analog that is modified intracellularly to its active form that can inhibit viral RNA-dependent RNA polymerases [34, 35], inhibiting the replication of the hepatitis C virus, enterovirus 71 [34, 35], and the human immunodeficiency virus [36]. Recently, it was discovered that oral azvudine led to the accumulation of the active form in the thymus in rats, inhibiting SARS-CoV-2 replication, preserving thymus immune function, and rapidly curing COVID-19 [21]. It also restored the expression of cytosine deaminase APOBEC3G, which is a single-stranded DNA cytidine deaminase that targets retroviral minus-strand DNA, with protective effects against retroviruses [37].
In the present study, the proportion of patients with moderate COVID-19 was lower in the SOC group compared with the azvudine and nirmatrelvir/ritonavir groups (8.7% vs. 92.9% vs. 83.3%). It was consistent with clinical practice since physicians were more likely to administer antiviral drugs to patients with more severe diseases. Despite the higher proportions of patients with moderate COVID-19, the patients in the azvudine and nirmatrelvir/ritonavir groups had remarkably shorter time from symptom onset to NANC than the SOC group (median, 14 vs. 15 vs. 19 days). Approximately one-fifth of the patients receiving azvudine achieved NANC within 7 days, while no patients in the nirmatrelvir/ritonavir or SOC groups achieved NANC within 7 days. The NANC rate within 10 days from admission was numerically higher in the azvudine group than in the other two groups (57.1% vs. 27.8% vs. 17.4%). Although the NANC rate within 14 days in the azvudine group was slightly lower than in the nirmatrelvir/ritonavir group, it was higher than in the SOC group (71.4% vs. 83.3% vs. 43.5%). Therefore, azvudine could reduce the viral load and accelerate the clearance of the SARS-CoV compared with SOC. Several studies could show that azvudine can reduce the time to NANC and improve the symptoms of patients with COVID-19, including those with comorbidities [21, 22, 38, 39], as supported by a meta-analysis [40]. Azvudine also appears more effective than nirmatrelvir/ritonavir in patients with comorbidities [41]. Although those studies did not specifically include older adults, they exclusively examined azvudine in patients with comorbidities. Older adults usually have comorbidities such as frailty, malnutrition, and various chronic diseases.
Of note, two patients in the SOC group progressed to severe COVID-19, while no patients in the azvudine or nirmatrelvir/ritonavir group progressed. Sun et al. [26] showed that patients with comorbidities (e.g., chronic obstructive pulmonary disease and active cancer) treated with azvudine had a lower rate of progression to severe COVID-19 compared with the patients without azvudine but without differences in mortality. Besides, the azvudine group had shorter LOS than the nirmatrelvir/ritonavir and SOC groups (median, 10.5 vs. 13.5 vs. 17 days), indicating that azvudine may help save medical resources. No TRAEs, SAEs, or impairment in liver or kidney functions were reported with azvudine, suggesting good safety. Hence, azvudine could be a treatment option for mild and moderate COVID-19 in older adults.
Older adults represent a special population of patients with COVID-19. Indeed, frailty and malnutrition are often encountered in older adults and are factors of poor prognosis for many diseases, including COVID-19 [42, 43]. In addition, several comorbidities observed in older adults are factors of poor COVID-19 prognosis, including cancer, cardiovascular diseases, kidney diseases, liver diseases, pulmonary conditions, and rheumatic and musculoskeletal diseases [10,11,12,13]. Previous studies in older adults with COVID-19 showed that nirmatrelvir/ritonavir could improve the symptoms [44] and shorten the NANC time [45, 46]. A study in the USA supported the use of molnupiravir or nirmatrelvir/ritonavir in patients ≥65 years of age [47]. Similar results were observed in the present study, with nirmatrelvir/ritonavir shortening the NANC time compared with SOC. Still, the present study also showed that azvudine might be superior to nirmatrelvir/ritonavir with regard to the NANC time. Of course, considering the non-randomized design and small sample size, formal head-to-head clinical trials would be required to demonstrate the superiority. The shortening of the NANC time by azvudine has been shown in various patient populations, including patients with mild [23] and mild-to-moderate COVID-19 [22]. Both nirmatrelvir/ritonavir and azvudine are better than conventional SOC in terms of shortening the NANC time in patients ≥80 years of age, and no patients progressed to severe diseases, supporting the effectiveness of antiviral drugs in this special patient population.
A relatively high risk of drug-drug interaction has been reported with nirmatrelvir/ritonavir [48], which is worth noticing in older adults who are often receiving various medications for comorbidities. Nirmatrelvir/ritonavir is reported to be associated with TRAEs such as nausea, diarrhea, headache, running nose, and muscle ache [49], and several more severe TRAEs of nirmatrelvir/ritonavir (bradycardia and syncope) have been observed recently [50]. Few TRAEs of azvudine were previously reported [22], mainly headache, dizziness, increased aminotransferases, nausea, and increased D dimer levels [23]. No TRAEs were noted in the azvudine or nirmatrelvir/ritonavir groups in the present study, suggesting the acceptable safety of these antiviral drugs. Of note, both drugs were only used for 5 days, and follow-up for long-term safety is needed.
A previous study showed the effectiveness of nirmatrelvir/ritonavir in lowering the mortality of patients with severe COVID-19 [51]. Nevertheless, as the virus mutates and its lethality decreases, it has become a major concern to control the disease progression of patients with mild-to-moderate COVID-19 and reduce hospitalization. For older adults, prolonged LOS and long COVID-19 infection are important risk factors for low quality of life. This study preliminarily explored the effectiveness of azvudine in shortening the NANC times and the LOS, therefore providing certain guidance for clinical medication and directions for future studies.
This study has limitations. First, due to the small sample size, this study had low statistical power, and only descriptive analyses were performed. Second, as a retrospective study, there might be information bias, especially regarding TRAEs. In addition, the data were limited to those available in the medical charts. Third, because of the limited medical resources during the Omicron outbreak in late 2022 in China, the admission of some patients might have been delayed, and the time from diagnosis to NANC might have been lengthened.
Conclusion
Azvudine showed satisfactory effectiveness and acceptable safety in older adults with mild or moderate COVID-19. Therefore, azvudine could be a treatment option for this special patient population.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CIs:
-
Confidence intervals
- LOS:
-
Length of hospital stay
- LHQW:
-
Lianhua Qingwen granules
- NANC:
-
Nucleic acid negative conversion
- SAEs:
-
Serious adverse events
- SOC:
-
Standard-of-care
- TCM:
-
Traditional Chinese medicine
- TRAEs:
-
Treatment-related adverse events
References
World Health Organization: Weekly epidemiological update on COVID-19 - 18 May 2023. Edition 143. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19%2D%2D-18-may-2023. Accessed May 22, 2023. Geneva: World Health Organization; 2023.
El-Sadr WM, Vasan A, El-Mohandes A. Facing the new Covid-19 reality. N Engl J Med. 2023;388:385–7.
Hammad HM, Nauman HMF, Abbas F, Jawad R, Farhad W, Shahid M, et al. Impacts of COVID-19 pandemic on environment, society, and food security. Environ Sci Pollut Res Int. 2023;30:99261-72.
Markov PV, Ghafari M, Beer M, Lythgoe K, Simmonds P, Stilianakis NI, et al. The evolution of SARS-CoV-2. Nat Rev Microbiol. 2023;21:361–79.
Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279–286.e278.
World Health Organization. Clinical management of COVID-19: living guideline. Geneva: World Health Organization; 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1. Accessed 22 May 2023
Chavda VP, Vuppu S, Mishra T, Kamaraj S, Patel AB, Sharma N, et al. Recent review of COVID-19 management: diagnosis, treatment and vaccination. Pharmacol Rep. 2022;74:1120–48.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–43.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–71.
Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52:1193–4.
Ge J, Pletcher MJ, Lai JC, Consortium NC. Outcomes of SARS-CoV-2 infection in patients with chronic liver disease and cirrhosis: a national COVID cohort collaborative study. Gastroenterology. 2021;161:1487–1501.e1485.
Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203:24–36.
Lebrasseur A, Fortin-Bedard N, Lettre J, Raymond E, Bussieres EL, Lapierre N, et al. Impact of the COVID-19 pandemic on older adults: rapid review. JMIR Aging. 2021;4:e26474.
Zhou Z, Zhang M, Wang Y, Zheng F, Huang Y, Huang K, et al. Clinical characteristics of older and younger patients infected with SARS-CoV-2. Aging (Albany NY). 2020;12:11296–305.
Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for covid-19. Bmj. 2020;370:m3379.
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral Nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–408.
Xie Y, Bowe B, Al-Aly Z. Nirmatrelvir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records. BMJ. 2023;381:e073312.
Zareifopoulos N, Lagadinou M, Karela A, Kyriakopoulou O, Velissaris D. Neuropsychiatric Effects of Antiviral Drugs. Cureus. 2020;12:e9536.
Kanai O, Fujita K, Nanba K, Esaka N, Hata H, Seta K, et al. Safety of Remdesivir for patients 80 years of age or older with coronavirus disease 2019 (COVID-19). Drugs Aging. 2021;38:1067–74.
Zhang J-L, Li Y-H, Wang L-L, Liu H-Q, Lu S-Y, Liu Y, et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6:414.
Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L, et al. A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv Sci (Weinh). 2020;7:e2001435.
da Silva RM, Gebe Abreu Cabral P, de Souza SB, Arruda RF, Cabral SPF, de Assis A, et al. Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front Med (Lausanne). 2023;10:1143485.
Kale A, Shelke V, Dagar N, Anders HJ, Gaikwad AB. How to use COVID-19 antiviral drugs in patients with chronic kidney disease. Front Pharmacol. 2023;14:1053814.
Izzedine H, Jhaveri KD, Perazella MA. COVID-19 therapeutic options for patients with kidney disease. Kidney Int. 2020;97:1297–8.
Sun Y, Jin L, Dian Y, Shem M, Zeng F, Chen X, et al. Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. eClinicalMedicine. 2023;59:101981.
Moghadas SM, Vilches TN, Zhang K, Wells CR, Shoukat A, Singer BH, et al. The impact of vaccination on COVID-19 outbreaks in the United States. medRxiv; 2021.
Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293–302.
Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J. Consortium C-GU, Peacock SJ, Barclay WS, de Silva TI, towers GJ, Robertson DL: SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21:162–77.
Rouzine IM, Rozhnova G. Evolutionary implications of SARS-CoV-2 vaccination for the future design of vaccination strategies. Commun Med (Lond). 2023;3:86.
Malik JA, Ahmed S, Mir A, Shinde M, Bender O, Alshammari F, et al. The SARS-CoV-2 mutations versus vaccine effectiveness: new opportunities to new challenges. J Infect Public Health. 2022;15:228–40.
Monto AS. The future of SARS-CoV-2 vaccination - lessons from influenza. N Engl J Med. 2021;385:1825–7.
Wheatley AK, Fox A, Tan HX, Juno JA, Davenport MP, Subbarao K, et al. Immune imprinting and SARS-CoV-2 vaccine design. Trends Immunol. 2021;42:956–9.
Klumpp K, Kalayanov G, Ma H, Le Pogam S, Leveque V, Jiang WR, et al. 2′-deoxy-4′-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2′-alpha-hydroxyl groups. J Biol Chem. 2008;283:2167–75.
Xu N, Yang J, Zheng B, Zhang Y, Cao Y, Huan C, et al. The pyrimidine analog FNC potently inhibits the replication of multiple enteroviruses. J Virol. 2020;94:10–128.
Wang RR, Yang QH, Luo RH, Peng YM, Dai SX, Zhang XJ, et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One. 2014;9:e105617.
Luo K, Wang T, Liu B, Tian C, Xiao Z, Kappes J, et al. Cytidine deaminases APOBEC3G and APOBEC3F interact with human immunodeficiency virus type 1 integrase and inhibit proviral DNA formation. J Virol. 2007;81:7238–48.
Deng G, Li D, Sun Y, Jin L, Zhou Q, Xiao C, et al. Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study. J Med Virol. 2023;95:e28756.
Chen W, Xu H, Hong L, Yang R, Peng C, Wang G, et al. Oral Azvudine (FNC) tablets in patients infected with SARS-CoV-2 omicron variant: a retrospective cohort study. medRxiv. 2023; 2023.01.05.23284180. https://www.medrxiv.org/content/10.1101/2023.01.05.23284180v1.article-info.
Chen Z, Tian F. Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis. Heliyon. 2023;9:e20153.
Dian Y, Meng Y, Sun Y, Deng G, Zeng F. Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities. J Inf Secur. 2023;87:e24–7.
McGovern J, Al-Azzawi Y, Kemp O, Moffitt P, Richards C, Dolan RD, et al. The relationship between frailty, nutritional status, co-morbidity, CT-body composition and systemic inflammation in patients with COVID-19. J Transl Med. 2022;20:98.
Hussien H, Nastasa A, Apetrii M, Nistor I, Petrovic M, Covic A. Different aspects of frailty and COVID-19: points to consider in the current pandemic and future ones. BMC Geriatr. 2021;21:389.
Zhang L, Zhang S, Han J, Yi Y, Zhou H, Li J. Paxlovid administration in elderly patient with COVID-19 caused by omicron BA.2.0: a case report. Medicine (Baltimore). 2022;101:e31361.
Lu G, Zhang Y, Zhang H, Ai J, He L, Yuan X, et al. Geriatric risk and protective factors for serious COVID-19 outcomes among older adults in Shanghai omicron wave. Emerg Microbes Infect. 2022;11:2045–54.
Zhong W, Yang X, Jiang X, Duan Z, Wang W, Sun Z, et al. Factors associated with prolonged viral shedding in older patients infected with omicron BA.2.2. Front. Public Health. 2022;10:1087800.
Gentry CA, Nguyen P, Thind SK, Kurdgelashvili G, Williams RJ. Characteristics and outcomes of US veterans at least 65 years of age at high risk of severe SARS-CoV-2 infection with or without receipt of oral antiviral agents. J Inf Secur. 2023;86:248–55.
Bihan K, Lipszyc L, Lemaitre F, Dautriche A, Fedrizzi S, Atzenhoffer M, et al. Nirmatrelvir/ritonavir (Paxlovid(R)): French pharmacovigilance survey 2022. Therapie. 2023;78:531-47.
Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19:a meta-analysis. Ann Med. 2022;54:516–23.
Ganipisetti VM, Bollimunta P, Maringanti S. Paxlovid-Induced Symptomatic Bradycardia and Syncope. Cureus. 2023;15:e33831.
Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023;76:e342–9.
Acknowledgments
Not applicable.
Funding
This study was funded by the Key Research & Developmental Program of Hunan Province (No. 2022SK2047 and 2020SK3014) and the Natural Science Foundation of Changsha City (No. Kp2208448).
Author information
Authors and Affiliations
Contributions
Zhiguo Zhou: experimental design, data statistics, and writing of the article; He Zheng: Ethical application, experimental procedure design, data statistics; Gui’e Xiao, Xiangping Xie: collected data and made statistics; Jiaxi Rang: experimental design, data statistics, and article writing; Danhong Peng: collected data, statistics, experimental design. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Central Hospital of Shaoyang (approval number: KY-2022-002-20). Informed consent was waived by the committee due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Z., Zheng, H., Xiao, G. et al. Effectiveness and safety of azvudine in older adults with mild and moderate COVID-19: a retrospective observational study. BMC Infect Dis 24, 47 (2024). https://doi.org/10.1186/s12879-023-08944-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08944-z