Abstract
Background
Imported cerebral malaria (CM) cases in non-endemic areas are often misdiagnosed, which delays treatment. Post-malaria neurological syndrome (PMNS) after recovery from severe malaria can also complicate diagnosis.
Case
We report an imported malaria case from West Africa with two sequential episodes with neurological syndromes within about a month. The first episode was diagnosed as CM with microscopy-positive Plasmodium falciparum infection. The second episode, occurring a month after the recovery from the first CM episode, was consistent with PMNS, since malaria parasites were not detected by microscopy in peripheral blood smears. However, this diagnosis was complicated by the detection of Plasmodium vivax in peripheral blood by PCR, suggesting a potential cause of the second episode by P. vivax.
Conclusion
This study suggests that PMNS often occurs after severe falciparum malaria. Concurrent P. vivax infection with pathogenic biomass being predominantly extravascular further complicates accurate diagnosis.
Similar content being viewed by others
Background
Cerebral malaria (CM) is the most common and serious neurological complication arising mostly from infection with P. falciparum [1, 2]. Mounting evidence also confirms P. vivax as another major cause of severe malaria despite relatively low-grade parasitemia in peripheral blood [3, 4]. The most common symptoms of severe vivax malaria include severe anemia, acute respiratory distress syndrome, impaired consciousness, and renal failure [5, 6]. Severe vivax malaria has been reported disproportionally from endemic countries [5]. Reviews of inpatient records in hospitals of western Cambodia and the Thailand-Myanmar border showed that severe vivax malaria, though relatively rare, was also an important cause of hospitalization [7, 8]. Likewise, vivax CM cases, although not frequently documented, have been reported mostly from India and Pakistan [5, 6, 9, 10]. Despite proper antimalarial treatment, CM still carries a 15–20% mortality rate in children and 30% in adults [11].
Since the symptoms of CM can deteriorate rapidly, timely diagnosis and effective treatment are vital for rescuing CM patients. Unfortunately, imported CM cases in non-endemic areas are often misdiagnosed, and delayed treatment leads to adverse and fatal outcomes. Here, we report an unusual malaria case in southern China imported from West Africa, where the patient experienced two sequential cerebral episodes within one month, which may be caused by microscopically patent P. falciparum and tenebrous P. vivax, respectively.
Case history
A 34-year-old male attended the general outpatient clinic of Shanglin County Hospital, Guangxi, China, on September 23, 2016 (Fig. 1A). The night before, the patient developed systemic pain and discomfort in the epigastric area. As his symptoms intensified by the morning, he sought medical attention at the hospital around noon. Because of his primary complaint of epigastric pain and no obvious abnormalities from the physical examination, an esophagogastroscopy was performed, but it did not identify any significant pathology except hyperemia and edema of the gastric mucosa. One hour post gastroscopy, the patient suddenly became confused and disoriented and was immediately referred to the emergency unit. Physical and laboratory examination revealed that the patient weighed 50 kg with a BMI of 18.4 kg/m2, slightly underweight. He was febrile (39.5℃), tachycardic (pulse rate of 111 times/min), and thrombocytopenic (Table 1).
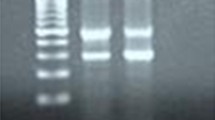
A Chronological events of the malaria case. The day of his first hospital admission was designated as Day 0. B Gel images of the PCR diagnosis of the patient’s two blood samples collected during his two hospital admissions targeting the 18S rRNA gene. M – molecular marker in bp; Pf – P. falciparum (205 bp); Pv – P. vivax (120 bp)
An accompanying family member provided medical information and travel history. The patient had worked in Ghana since August 2014 and returned to his home in China ten days before the presentation. During his stay in Ghana, he did not take chemoprophylaxis, had suffered from malaria several times, and had been treated locally. The routine malaria screen by microscopy at the County CDC 7 days before his presentation (September 17, 2016) was Plasmodium-negative. Given his travel history and symptoms, he was suspected of having a malaria infection. Microscopy of his blood smear confirmed P. falciparum infection with a parasite density of 3.2×104/μl. With the diagnosis of CM, rescue treatment with intravenous artesunate (loading dose of 120 mg, and subsequently 60 mg/4 h) was immediately initiated. Five hours after admission, he was in a coma and unresponsive, with a Glasgow coma score of 4 (No eye opening, +1; no verbal response, +1; extend motor response, +2). He exhibited shallow breathing and had coffee-colored urine. Blood LDH level and urinalysis also indicated massive hemolysis (Table 1). He was transferred to the intensive care unit and rapidly required intubation for mechanical ventilation. At 12 h, he remained thrombocytopenic with elevated lactic acid dehydrogenase and bilirubin (Table 1). Parasitological monitoring every 4-8 h showed that his parasitemia continually decreased during the treatment. At 44 h, the patient became aparasitemic, slightly febrile (37.5°C), and more alert. The mechanical ventilation was weaned, and artesunate injection was adjusted to 60 mg/12 h. At 66 h, he returned to his baseline mental status and was transferred to the inpatient unit, where his condition continued improving. Seven days after admission, he was discharged and prescribed a full course of dihydroartemisinin-piperaquine oral tablets for the subsequent three days. Seven days later, a blood examination showed that he was parasite-negative and had made a good recovery (Table 1).
At 1:00 pm on October 26, 2016, he collapsed while working at home. He developed generalized seizures and became unresponsive (Fig. 1A). He was rushed to the county hospital. Physical, routine laboratory and head CT examinations revealed no obvious abnormalities (Table 1). He was unconscious with a Glasgow coma score of 4, lethargic, and irritable. Microscopy of peripheral blood thick smear (400 ×, 100 fields) showed no Plasmodium. The patient developed a fever (38.5℃) at 7:30 pm, following which he was presumptively administered intravenous artesunate because of possible malaria recrudescence. The next morning (4:30), the patient became afebrile and regained consciousness. Two days after admission, the patient's vital signs returned normal. Antimalarial therapy was discontinued, and blood smears examined every 8 h were Plasmodium-negative. Five days after admission, the patient was discharged with a course of artesunate-amodiaquine oral tablets.
The patient's blood samples at the two admissions were sent to Kunming Medical University for molecular diagnosis. Nested PCR [12] detected mixed P. falciparum/P. vivax infections during his first episode and P. vivax for the second (Fig. 1B). With a normal glucose-6-phosphate dehydrogenase level, he received a course of oral treatment with chloroquine (1500 mg/3 days) and primaquine (22.5 mg/day for eight days) beginning 11 days after his second episode to prevent future relapse. He remained healthy throughout a two-year follow-up.
Discussion
Prompt diagnosis and treatment of malaria are challenging in non-endemic countries, and delays are associated with increased risks of severe disease and mortality [13]. In our case, travel history played a critical role in the diagnosis. Since malaria was eliminated in Guangxi in 2012 [14] and the patient presented with atypical malaria symptoms at his first admission, diagnosis for malaria was delayed until his symptoms rapidly deteriorated to a comatose condition. It was his African travel history that alerted the doctors and led to the diagnosis of P. falciparum CM. Since 2012, treating thousands of returning expatriates with imported malaria infection from Africa provided adequate training to the medical doctors in the Shanglin County Hospital. Increased awareness and preparedness of doctors regarding early detection and treatment of imported malaria in non-endemic countries are desired.
Compared to the WHO definition of CM, which requires the identification of asexual Plasmodium parasitemia in peripheral blood, the second episode of neurological syndrome resembles the post-malaria neurological syndrome (PMNS), a complication occurring within two months after recovery from a severe P. falciparum attack without detecting malaria parasites in the blood [15,16,17]. PMNS is a predominantly self-limiting condition with a median duration of symptoms of 13 days (range 3-25 days) [16]. However, this diagnosis was complicated by the detection of P. vivax by PCR, which suggested that the second episode might be due to P. vivax infection. It could be argued that the PCR result for the second episode might be from the persisting parasite DNA from the first episode of active infection, but the PCR detection of P. vivax but not P. falciparum (the predominant infection during the first episode) argues in favor of active malaria. Besides, the patient responded rapidly to artesunate without antibiotic treatment (compared to the relatively slower process of PMNS), suggesting Plasmodium and excluding bacterial meningitis as a probable cause of the second cerebral episode. Thus, despite the patient only had submicroscopic P. vivax parasitemia in peripheral blood, the fact that P. vivax may have pathogenic biomass that is predominantly extravascular may explain his second episode, which is consistent with the definition of severe tenebrous vivax malaria with cerebral syndrome [18].
This case demonstrates the risk of both P. falciparum and P. vivax as the cause of severe malaria in travelers to Africa. Since coma associated with P. vivax mono-infection is much less frequent than falciparum malaria [19], the finding of imported P. vivax infection from West Africa as the potential underlying cause of his second episode is unexpected but consistent with the increasing detection of vivax malaria in Duffy– populations. Whereas P. vivax was not detected in surveys in Ghana [20], reports of P. vivax infections in travelers/migrants from Ghana suggest the existence of P. vivax transmission there [14, 21]. In addition, with the hidden reservoir of P. vivax in extravascular space, a combination of microscopy with serological and molecular tests is needed to diagnose P. vivax infections in returning travelers. As imported relapsing malaria incidence continues to rise in non-endemic regions [14], this case illustrates the need for heightened awareness among healthcare providers to consider P. vivax alone or as a mixed infection with P. falciparum in the absence of detectable vivax parasitemia as the potential cause in patients with a travel history and altered mental status.
This case report has revealed several limitations with the current malaria diagnosis and treatment policy in China. The lack of a comprehensive febrile diagnostic work-up and reliance on microscopy for malaria diagnosis will certainly overlook non-malarial infections and submicroscopic malaria, as presented in this case. Notably, the severe malaria case was treated with artesunate infusion with a shortened interval. Despite promising results from treating many severe malaria cases in Shanglin Hospital, the regimen requires vigorous clinical studies. In addition, uncomplicated falciparum malaria in China is treated with an artemisinin-based combination therapy without a single low-dose primaquine. Although this may appear justified for imported malaria in areas without vectors, adding the low-dose primaquine should be advocated, especially in southern China, where malaria has recently been eliminated, and vectors are abundantly present.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding authors upon reasonable request.
References
Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–9.
Luzolo AL, Ngoyi DM. Cerebral malaria. Brain Res Bull. 2019;145:53–8.
Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25:220–7.
Baird JK. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev. 2013;26:36–57.
Rahimi BA, Thakkinstian A, White NJ, Sirivichayakul C, Dondorp AM, Chokejindachai W. Severe vivax malaria: a systematic review and meta-analysis of clinical studies since 1900. Malar J. 2014;13:481.
Kotepui M, Kotepui KU, Milanez GJ, Masangkay FR. Prevalence and risk factors related to poor outcome of patients with severe Plasmodium vivax infection: a systematic review, meta-analysis, and analysis of case reports. BMC Infect Dis. 2020;20:363.
Lon C, Timmermans A, Buathong N, Nou S, Se Y, Sitthy N, Chann S, Kraesub S, Wongstitwilairoong T, Walsh DS, et al. Severe malaria in Battambang Referral Hospital, an area of multidrug resistance in Western-Cambodia: a retrospective analysis of cases from 2006–2009. Malar J. 2013;12:217.
Chu CS, Stolbrink M, Stolady D, Saito M, Beau C, Choun K, Wah TG, Mu N, Htoo K, Nu B, et al. Severe falciparum and vivax malaria on the Thailand-Myanmar border: a review of 1503 cases. Clin Infect Dis. 2023;77:721–8.
Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA Jr. Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg. 2002;67:230–2.
Kojom Foko LP, Arya A, Sharma A, Singh V. Epidemiology and clinical outcomes of severe Plasmodium vivax malaria in India. J Infect. 2021;82:231–46.
Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Taylor TE. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9:1173–81.
He X, Pan M, Zeng W, Zou C, Pi L, Qin Y, Zhao L, Qin P, Lu Y, Baird JK, et al. Multiple relapses of Plasmodium vivax malaria acquired from West Africa and association with poor metabolizer CYP2D6 variant: a case report. BMC Infect Dis. 2019;19:704.
Leder K, Black J, O’Brien D, Greenwood Z, Kain KC, Schwartz E, Brown G, Torresi J. Malaria in travelers: a review of the GeoSentinel surveillance network. Clin Infect Dis. 2004;39:1104–12.
Liu P, Shen L, Wang S, Qin P, Si Y, Pan M, Zeng W, Qin Y, Chen X, Zhang Y, et al. Increasing proportions of relapsing parasite species among imported malaria in China’s Guangxi Province from Western and Central Africa. Travel Med Infect Dis. 2021;43:102130.
Nguyen TH, Day NP, Ly VC, Waller D, Mai NT, Bethell DB, Tran TH, White NJ. Post-malaria neurological syndrome. Lancet. 1996;348:917–21.
Yadava SK, Laleker A, Fazili T. Post-malaria neurological syndrome: a rare neurological complication of malaria. Infection. 2019;47:183–93.
Markley JD, Edmond MB. Post-malaria neurological syndrome: a case report and review of the literature. J Travel Med. 2009;16:424–30.
Baird JK. African Plasmodium vivax malaria improbably rare or benign. Trends Parasitol. 2022;38:683–96.
Lampah DA, Yeo TW, Hardianto SO, Tjitra E, Kenangalem E, Sugiarto P, Price RN, Anstey NM. Coma associated with microscopy-diagnosed Plasmodium vivax: a prospective study in Papua Indonesia. PLoS Negl Trop Dis. 2011;5:e1032.
Brown CA, Pappoe-Ashong PJ, Duah N, Ghansah A, Asmah H, Afari E, Koram KA. High frequency of the Duffy-negative genotype and absence of Plasmodium vivax infections in Ghana. Malar J. 2021;20:99.
Quaye IK, Aleksenko L, Oeuvray C, Yewhalaw D, Duah N, Gyan B, Haiyambo DH, Dongho GBD, Torgby RA, Amoah L, et al. The Pan African Vivax and Ovale Network (PAVON): Refocusing on Plasmodium vivax, ovale and asymptomatic malaria in sub-Saharan Africa. Parasitol Int. 2021;84:102415.
Acknowledgments
Thanks the participant for consenting to the case for publication and the staff of Kunming Medical University, Shanglin County People's Hospital for overall support.
Funding
This study was supported by the National Science Foundation of China (31860604 and U1802286) and the National Institutes of Health, USA (U19AI089672). YZ was also supported by the Major science and technology projects of Yunnan Province (2018ZF0081) and International Science and Technology Cooperation-Yunnan International Science and Technology Cooperation Base (202003AE140004). ZX was sponsored by the Yunnan Applied Basic Research Projects-Union Foundation (2019FE001-015). The human subject study was approved by the institutional review board of Shanglin County People’s Hospital.
Author information
Authors and Affiliations
Contributions
ZX and LZ collect data and wrote the original manuscript. MP, YQ, YB, PQ, WZ, XW, YL collect data and rescue the patient. CS, LM, YH, LC, ZYwrite the paper. All authors have read and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Kunming Medical University (No. KMMU2021MEC110). The patient has consent to participate in our research.
Consent for publication
Written informed consent was obtained from the participant and their legal guardians for publication of identifying information/images in an online open-access publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Sup Fig. 1.
Agarose gel images of the PCR diagnosis of the patient’s blood samples collected during his two hospital admissions targeting the 18S rRNA gene. M – molecular marker in bp; Pf – P. falciparum (205 bp); Pv – P. vivax (120 bp).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xiang, Z., Zhou, L., Pan, M. et al. An imported malaria case with repeated episodes of neurological syndromes resulting from different Plasmodium species. BMC Infect Dis 24, 41 (2024). https://doi.org/10.1186/s12879-023-08872-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08872-y