Abstract
Background
Respiratory tract infections (RTIs) are a major global health burden due to their high morbidity and mortality. This retrospective study described the epidemiology of respiratory pathogens in adults over a 5-year period at an Australian tertiary healthcare network.
Methods
All multiplex reverse transcription polymerase chain reaction respiratory samples taken between the 1st of November 2014 and the 31st of October 2019 were included in this study. Overall prevalence and variations according to seasons, age groups and sex were analysed, as well as factors associated with prolonged hospital and intensive care length of stay.
Results
There were 12,453 pathogens detected amongst the 12,185 positive samples, with coinfection rates of 3.7%. Picornavirus (Rhinovirus), Influenza A and respiratory syncytial virus were the most commonly detected pathogens. Mycoplasma pneumoniae was the most commonly detected atypical bacteria. Significant differences in the prevalence of Chlamydia pneumoniae and Human metapneumovirus infections were found between sexes. Longest median length of intensive care and hospital stay was for Legionella species. Seasonal variations were evident for certain pathogens.
Conclusions
The high rates of pathogen detection and hospitalisation in this real-world study highlights the significant burden of RTIs, and the urgent need for an improved understanding of the pathogenicity as well as preventative and treatment options of RTIs.
Similar content being viewed by others
Summary
Distinct trends of respiratory pathogens observed across seasons, age groups and sex contribute to a large burden of disease during the pre-COVID-19 era. Of viral pathogens, Picornavirus, influenza A and respiratory syncytial virus were the most common viral pathogens whilst Mycoplasma pneumoniae was the most common atypical bacterial pathogen detected on multiplex reverse transcription polymerase chain reaction.
Introduction
Respiratory tract infections (RTIs) are a major global health burden due to their high morbidity and mortality. They accounted for approximately 336.5 million infections and 2.4 million deaths in a 2016 global burden of disease estimate [1, 2]. Although most RTIs are self-limiting, they can contribute to severe outcomes including hospitalisation and death [1]. This is particularly seen in young children, the elderly and those who are immunocompromised [3, 4]. In Australia, RTIs account for up to 7 million visits to general practitioners each year, with an average individual experiencing 2 to 5 episodes of infection per year [5]. Due to their high prevalence, RTIs place an enormous burden on healthcare systems, imposing a significant economic cost in terms of direct medical expenses including hospitalisation, primary care reviews and antibiotic prescriptions as well as indirect productivity losses [6].
The majority of RTIs are caused by viruses, followed by bacterial infections [7, 8]. Due to the undifferentiated nature of respiratory symptoms at presentation between pathogen type/s, diagnostic uncertainty contributes to the mounting costs of investigations and antibiotic overprescribing [9]. Multiplex reverse transcription polymerase chain reaction (mPCR) allows the rapid detection of a panel of respiratory pathogens (inclusive of both viruses and bacteria) and identifies coinfection in a single test [10, 11]. mPCR has largely replaced previous testing methods, including cultures, which were less sensitive, have limited ability for pathogen detection and are more time consuming. Whilst mPCR allows for more tailored treatment for patients, on a larger scale, it is also useful in the monitoring of epidemiological trends of RTIs [12].
In recent times, interest in respiratory pathogens has increased due to the emergence of several novel viruses. These include severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19 illness and influenza A virus subtype H5N1 [12, 13]. Up to 30% of adults admitted to hospital with community acquired pneumonia (CAP) prior to the COVID-19 pandemic had a viral aetiology [14], with an overall mortality of 3% [15] and over 15–20% of severe CAP cases in adults were attributed to influenza alone [16]. Furthermore, respiratory syncytial virus (RSV) is increasingly being recognized as a cause of illness in high-risk adults, including those with chronic cardiorespiratory disease, and those who are immunocompromised [17] or older [18]. Such patient groups are particularly at risk of severe infection, with intensive care unit (ICU) admissions and mortality rates being similar to that of influenza [19, 20]. Currently, available vaccines only target against Streptococcus pneumoniae, Haemophilus influenzae B, influenza A and B, and COVID-19.
Both influenza and pneumococcal epidemiology is well defined through surveillance systems [21,22,23]. The influenza season is known to coincide with the cooler months in Australia, from May to October each year (end of autumn to mid-spring), with the remaining six months defined as non-influenza season [24]. However, seasonal variations of other respiratory pathogens are less studied in the temperate southern hemisphere. An improved understanding of the epidemiology of RTIs pathogens may help manage seasonal outbreaks and individual patients more effectively. Furthermore, variations according to host factors, including age and sex, are not well understood in the Australian context, nor are the impacts of RTIs on healthcare resource utilisation such as hospital length of stay (LOS) in either internal medicine wards (IMWs) or ICUs.
The objective of this retrospective study was to describe the overall prevalence of non-pneumococcal respiratory pathogens in Australian adults at a single healthcare network between 2014 to 2019, and identify variations in season, sex and age that were associated with ICU admission and IMW LOS. Considering the paucity of Australian data on RTI epidemiology and pathogens, this information may assist in bettering the understanding of outbreaks as well as healthcare resource allocation.
Methods
Data for this retrospective study was collected across 3 of Monash Health’s acute hospitals in metropolitan Melbourne, Australia. Monash Health is a public health network that serves as a catchment for over one quarter of the state’s 6.6 million residents and has 1,389 beds across the 3 sites. There are 20 acute public hospitals in Metropolitan Melbourne, with 10 having ICUs. Most of the healthcare in Australia is provided in a public setting with less than 30% of patients optionally presenting to private hospitals [25]. In Australia, all citizens and permanent residents can receive universal health coverage under the Medicare scheme. This includes access to mPCR testing, as indicated by the presence of respiratory tract infection symptoms in those receiving care in a hospital setting. Data from all adult patients with a respiratory mPCR result dated between the 1st of November 2014 and the 31st of October 2019 were included in the study. Samples were obtained by the treating physicians when clinical indications (e.g. respiratory symptoms, systemic symptoms, radiographic changes, etc.) were present on presentation to ED or within 48 h of inpatient admission. Samples were collected from the upper and/or lower respiratory tracts. Upper respiratory tract samples were defined as any sample taken from the nose, throat or nasopharynx using standardised nasopharyngeal swab procedure across Monash Health’s sites. Lower respiratory tract samples included spontaneously expectorated sputum, endotracheal or bronchoscopic specimens. Samples were processed at the microbiological laboratory onsite at the hospital it was collected at; Casey Hospital, Dandenong Hospital or Monash Hospital, respectively. Samples were processed using AusDiagnostics High-Plex Respiratory Pathogens Assay (New South Wales, Australia) according to the manufacturer’s instructions. The assay was used to detect the following 9 viruses and 4 atypical bacteria: Adenovirus, Human metapneumovirus (HMPV), influenza A and B, parainfluenza virus 1, 2 and 3 (PIV 1, 2 and 3), Picornavirus, RSV, Bordetella spp, Chlamydia pneumoniae, Legionella spp, and Mycoplasma pneumoniae. The assay was unable to detect Bordetella spp. and Legionella spp until after 2015 or distinguish Rhinovirus from Enterovirus; hence these were grouped together as Picornaviruses. For this study, we assumed that almost all Picornaviruses were Rhinoviruses given the clinical context. Data was retrospectively retrieved from patient files via the Monash Health electronic medical records system.
Statistical analysis
The distribution of respiratory pathogens was analysed by age, sex and season. The four seasons in the southern hemisphere are: summer (December-February), autumn (March-May), winter (June-August) and spring (September-November). The age of patients was stratified into 3 categories as is common in clinical practice: 18–20 years, 20–70 years, and elderly patients, as per the ASPREE study’s definition being > 70 years [21]. LOS data was categorised by virus type as well as by nature of hospital admission. Duplicate samples were defined as positive samples taken from the same patient within a 30-day period from the initial positive test and were not included in the analysis. Since the samples were obtained from a single healthcare network, site specific distribution of samples was not analysed.
Categorical variables were presented as counts and proportions. Continuous variables were assessed for normality and summarised using mean and standard deviation (SD) or median and interquartile range (IQR) according to data type and distribution. Group comparisons were performed using chi-square test for equal proportions or Fisher’s exact test where numbers were small. A two-sided p value < 0.05 was considered statistically significant. Statistical analysis was performed using the SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient demographics
Figure 1 depicts sample size and specimen origin data. Of the 46,350 samples, 17,540 were positive for at least one pathogen. Once duplicates were removed from the dataset, there were 12,185 (29.7%) samples that were suitable for inclusion in the data analysis. The mean (SD) age of patients with a positive sample was 62.0 (21.2) years, and 6,408 patients (52.6%) were female. The frequency of pathogens across both sexes is displayed in Table 1. Statistically significant differences in RTI frequency was seen between the sexes for HMPV and C. pneumoniae with more cases being seen in females (HMPV 9.2% vs. 7.6%, p = 0.002) and males (C. pneumoniae 0.8% vs. 0.5%, p = 0.006), respectively.
Prevalence of respiratory pathogens
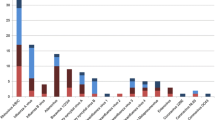
Across the 12,185 positive samples, a total of 12,453 pathogens were identified (12,208 [98%] viruses and 245 [2%] bacteria). The prevalence of respiratory pathogens across the study period is presented in Table 1, with Picornavirus (32.9%), Influenza A (28.7%) and RSV (11.1%) being the most prevalent. M. pneumoniae was the most common atypical bacteria detected (0.8%).
Respiratory pathogen co-infection
The singular and co-infection rates are outlined in Table 2, with a breakdown of viral versus bacterial aetiology. The three most common viruses detected in dual-positive samples were Picornavirus (48.6%), influenza A/B (47.3%), and RSV (24%). In triple-positive samples, these were influenza A/B (90%), RSV (40%) and C. pneumoniae (30%).
Dual infections were seen in all age groups, with almost a third occurring in individuals > 70 years old. In contrast, triple infections were only detected in 10% of individuals > 70 years, with the majority (50%) found in those over 40 years of age.
Sample collection location
Of the 12,185 samples, 8,876 samples were collected in ED, 797 in ICU and the remaining 2,512 on IMWs.
Relationship between respiratory pathogens and age
The prevalence of each pathogen type across the three categories of age is shown in Table 3.
Seasonal distribution of different respiratory pathogens
Seasonal variations for respiratory pathogens studied are shown in Fig. 2. While the majority of pathogens followed distinct seasonal patterns, the trends of the three most common pathogens (Picornavirus, influenza A, and RSV) are as follows. Picornavirus circulated all year round, with bimodal peaks in autumn and late spring, and a trough in mid-late summer. Influenza A showed seasonal peaks in late winter and early spring and had a similar number of positive samples to Picornavirus in spring. Lastly, RSV showed a seasonal pattern, with the highest number of presentations being in early winter.
Hospitalisation duration for respiratory pathogens
Ten thousand three hundred twenty-six (86.5%) patients were managed on internal medicine wards (IMWs), and 797 (6.7%) in ICUs. Picornavirus (IMW n = 3383, ICU n = 320), influenza A (IMW n = 2788, ICU n = 167) and RSV (IMW n = 1160, ICU n = 86), respectively, accounted for the most hospital admissions. Median LOS for ED, ICU and IMW hospitalisation according to different RTI pathogens are presented in Table 4. The median length of stay in each department accounting for all pathogens was 7.1 h in ED, 4.5 days in ICU and 3.6 days in IMWs.
Discussion
To date, there have been few large-scale Australian studies examining the epidemiology of respiratory pathogens in individuals presenting to acute hospitals. In this retrospective study, we found a modest positivity rate of 29.7% for at least one respiratory pathogen. Our results are slightly lower than the 30–45% positivity rate reported by other studies that utilised mPCR testing of nasopharyngeal samples [5, 22]. Our lower detection rate may relate to excluding duplicate positive results for the same individual, as well as inclusion of lower respiratory tract samples, while other studies did not.
Overall, Picornavirus (predominantly Rhinovirus) was the most frequently detected pathogen. This particularly affected the 20–70 year old age group, which is consistent with findings of previous publications [24, 26]. Previously, picornaviruses were thought to represent a mild disease, however there is now increasing evidence that picornaviruses play an important role in exacerbations of chronic respiratory diseases and contribute to morbidity and mortality [27]. Recent data also suggest an increasing association between Picornavirus and severe hospital and community acquired pneumonia [9, 28], with one study reporting higher mortality in adults hospitalised with rhinovirus when compared with influenza [29]. In our study, not only was Picornavirus the most prevalent RTI pathogen, it also contributed to the highest hospitalisation rate, thus reflecting its importance as an emerging health threat and highlighting the need for urgent action, given there are limited vaccine and therapeutic options against it.
Influenza A was found to be the second most common RTI pathogen. It demonstrated the expected seasonal pattern of having highest case numbers in winter and spring. This seasonality has been reflected in temperate regions around the world, while tropical regions tend to have a more diverse outbreak pattern [30]. In our cohort, influenza A had one of the shortest ICU LOSs, and a modest LOS in IMWs, despite being the second most common pathogen causing hospitalisation. Although previous literature is scarce, it notes that the average duration of hospitalisation for influenza is between 6.5 and 8.3 days [31] and that 6% of patients require care in the ICU [32]. This is similar to our rate of 5.9%. In one of the only prospective studies in this field, Thompson et al. determined that there is a reduction in ICU LOS and a 59% reduction in the risk of an ICU stay for those that are vaccinated for influenza [33]. Under the Australian National Immunisation Program, it is estimated that up to 70% of those aged above 75 years receive the influenza vaccine annually [34]. However, despite the eligibility criteria for influenza vaccination at no cost, vaccine uptake by patients with ‘at-risk’ conditions remains variable, thus opportunities still exist to improve vaccination rates to lessen the disease burden [35].
We observed RSV to be the third most prevalent virus in our study, with peak numbers in autumn and winter. As expected, higher positivity rates were seen in those > 70 years old compared to younger age groups in this study. Commonly associated with bronchiolitis and pneumonia in children, RSV is now recognised as an important pathogen in adults, especially in the elderly and those with comorbid cardiorespiratory disease [36]. Severe complications such as respiratory failure, prolonged hospitalisation and mortality rates similar to seasonal influenza have been observed in adults admitted to hospital with RSV infections. Interestingly, the risk of death has been reported to be higher from RSV than influenza after adjusting for comorbidities [37]. Our data demonstrates that the median LOS for IMWs and ICU for RSV was higher than influenza A. Although the use of RSV-specific immunoglobulins, palivizumab and ribavirin, have been studied in infants, there is limited data regarding their efficacy in adults, with the mainstay of treatment being symptomatic management [16]. Given the frequency of RSV detection in hospitalised patients and the poor outcomes for certain patient groups, the unmet need for antiviral and immunoglobulin therapy and vaccination against RSV in adults should be promptly explored. Furthermore, exploring the efficacy of inpatient isolation is a simple measure that may be highly effective in the containment of cases.
M. pneumoniae was a common cause of respiratory tract infections before the COVID-19 pandemic, with worldwide incidence of 8.6% from 2017 to 2020, measured by direct test methods [38]. The incidence reduced to 1.7% between 2020 and 2021 [38]. A study by Sauteur et al. (2023) comments on the scarcity of M. pneumoniae in the post-pandemic world, which is thought to be a result of the non-pharmaceutical interventions utilised to prevent COVID-19 transmission. We note that in our pre-COVID pandemic dataset, M. pneumoniae was the most commonly detected atypical bacteria. Thus, it is important for future Australian studies to monitor the prevalence of the disease, with particular attention being paid to the possibility of resurgence in a population potentially lacking immunity.
Our co-infection rate of 3.7% was higher than the results reported by Vissaeux et al. who found an incidence of 1.6% in 7,196 samples over 5 years [12]. A reason for this could lie in geographic differences and host factors such as immunosuppression which we did not explore in this study. Interestingly, within the constraints of the bacterial infections that we tested for via mPCR, we found that the majority of coinfections in our study included viruses only, with few coinfections including bacterial pathogens. Previous studies suggest that some viruses, particularly rhinoviruses, are able to reduce the ability of other viruses to establish infection via viral-viral interference, whilst the opposite is true for other pathogens [39,40,41]. This would suggest that there would be less viral coinfections. However, the difference in results may be explained by the limited number of bacterial pathogens that were possible to be detected via our mPCR panel.
Ultimately, our study has several limitations. Firstly, the cohort sampled was those who presented or were referred to a tertiary hospital for any cause. Such referral bias may not be a true reflection of a random sample of the broader Australian community. Secondly, we did not explore the clinical data, hence, a more accurate estimation of LOS directly attributable to individual pathogens was not possible. Additionally, risk factors such as immunocompromised status may further contribute to the aetiology of infection and LOS. Lastly, our mPCR assay did not detect other respiratory viruses such as coronavirus and bocavirus, was incapable of distinguishing enterovirus from rhinovirus as well as the H1N1 and H5N1 strains of influenza A, and we did not include various other bacterial infections such as pneumococcus or those that could be detected/isolated by antigens, microscopy and cultures in our data collection. Consequently, any differential distribution or pathogenicity could not be assessed, and our co-infection prevalence may be underreported.
Since the introduction of public health measures, which include social distancing and mask wearing to curb the spread of COVID-19 between 2019 to 2022, it has been noted that there has been a major decrease in the prevalence of all respiratory viral infections [42, 43]. On a global scale, influenza circulation was particularly low in 2020 as was demonstrated by near complete elimination in Taiwan [44] and significantly lower case numbers in various other countries [21]. Whilst a resurgence started in late 2021, in the first part of 2022 with easing of some of public health measures, seasonal patterns had not returned to normal in the Northern or Southern hemisphere [23]. Over the coming months and years, it will be an imperative to monitor if trends for both influenza and other pathogens return to that of something similar to pre-pandemic times, or if new patterns of RTI pathogens arise, especially in relation to climate change.
In summary, we have provided a broad snapshot of the epidemiology and healthcare utilisation for patients presenting to hospital with acute RTIs over a five-year period in Melbourne, Australia. The high rates of non-pneumococcal aetiology of RTI positivity obtained in this real-world study highlights the significant burden of infection, dominated by Picornavirus, influenza A and RSV, and the urgent need for preventative and treatment options. Furthermore, in seeing patterns of RTIs and the burden they place on the healthcare system, funding can be guided towards appropriate measures to improve healthcare utilisation, as well as reduce spread of disease, mortality and morbidity. In turn, this will prevent loss of productivity, improve individuals’ health and quality of life, and decrease healthcare expenditure. Future research is needed to improve our understanding of the pathogenicity of these viruses and note new trends in epidemiology post COVID-19 lockdowns and due to impact of climate change.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Troeger C, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower Respiratory Infections in 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–210.
Yu J, et al. Comparison of the prevalence of respiratory viruses in patients with acute Respiratory Infections at different hospital settings in North China, 2012–2015. BMC Infect Dis. 2018;18(1):72.
Echavarría M, et al. Clinical impact of rapid molecular detection of respiratory pathogens in patients with acute Respiratory Infection. J Clin Virol. 2018;108:90–5.
Nicholson KG, et al. Acute viral Infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of Disease burden. BMJ. 1997;315(7115):1060–4.
Chen Y, Kirk MD. Incidence of acute Respiratory Infections in Australia. Epidemiol Infect. 2014;142(7):1355–61.
Kotwani A, Holloway K. Antibiotic prescribing practice for acute, uncomplicated respiratory tract Infections in primary care settings in New Delhi, India. Trop Med Int Health. 2014;19(7):761–8.
Green DA, et al. Clinical utility of On-Demand multiplex Respiratory Pathogen Testing among adult outpatients. J Clin Microbiol. 2016;54(12):2950–5.
Jain S, et al. Community-Acquired Pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–45.
Harbarth S, Samore MH. Antimicrobial resistance determinants and future control. Emerg Infect Dis. 2005;11(6):794–801.
Chen Y, et al. Simultaneous detection of Influenza A, Influenza B, and respiratory syncytial viruses and subtyping of Influenza A H3N2 virus and H1N1 (2009) virus by multiplex real-time PCR. J Clin Microbiol. 2011;49(4):1653–6.
Visseaux B, et al. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLoS ONE. 2017;12(7): e0180888.
Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus Infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory Illness. J Clin Microbiol. 2002;40(3):817–20.
Sangil A, et al. Aetiology of community-acquired Pneumonia among adults in an H1N1 pandemic year: the role of respiratory viruses. Eur J Clin Microbiol Infect Dis. 2012;31(10):2765–72.
Ishiguro T, et al. Etiology and factors contributing to the severity and mortality of community-acquired Pneumonia. Intern Med. 2013;52(3):317–24.
Mermond S, et al. Aetiology of community-acquired Pneumonia in hospitalized adult patients in New Caledonia. Tropical Med Int Health. 2010;15(12):1517–24.
Eden J-S, et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun. 2022;13(1):2884.
Falsey AR, et al. Respiratory syncytial virus Infection in Elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–59.
Fleming DM, et al. Modelling estimates of the burden of respiratory Syncytial virus Infection in adults and the elderly in the United Kingdom. BMC Infect Dis. 2015;15:443.
Lee N, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus Infections. Clin Infect Dis. 2013;57(8):1069–77.
National Notifiable Diseases Surveillance System (NNDSS) public dataset – pneumococcal disease (invasive). Available from: https://www.health.gov.au/resources/publications/nndss-public-dataset-pneumococcal-disease-invasive#:~:text=This%20National%20Notifiable%20Diseases%20Surveillance,year's%20notifications%20and%20all%20updates.
McNeil JJ, et al. Effect of aspirin on all-cause mortality in the healthy Elderly. N Engl J Med. 2018;379(16):1519–28.
Zachariah P, et al. Community -and hospital laboratory-based surveillance for respiratory viruses. Influenza Other Respir Viruses. 2016;10(5):361–6.
Australian influenza surveillance report and activity update. Available from: https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-ozflu-flucurr.htm.
Atmar RL, et al. Picornavirus, the most common respiratory virus causing Infection among patients of all ages hospitalized with acute respiratory Illness. J Clin Microbiol. 2012;50(2):506–8.
Available from: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/dec-2019#states-and-territories.
Greenberg SB. Respiratory consequences of Rhinovirus Infection. Arch Intern Med. 2003;163(3):278–84.
Edwards MR, Ritchie AI, Johnston SL. Exacerbations of chronic respiratory diseases. Rhinovirus Infect. 2019;137–68. https://doi.org/10.1016/B978-0-12-816417-4.00006-8. Epub 2019 Jul 11.
Choi SH, et al. Viral Infection in patients with severe Pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186(4):325–32.
Hung IF, et al. Unexpectedly higher morbidity and mortality of hospitalized elderly patients associated with rhinovirus compared with influenza virus respiratory tract infection. Int J Mol Sci. 2017;18(2):259.
Tamerius JD, et al. Environmental predictors of seasonal influenza epidemics across temperate and tropical climates. PLoS Pathog. 2013;9(3): e1003194.
Pormohammad A, et al. Comparison of Influenza type A and B with COVID-19: a global systematic review and meta-analysis on clinical, laboratory and radiographic findings. Rev Med Virol. 2021;31(3): e2179.
Beatty K, Hamilton V, Kavanagh PM. Just a bad Flu? Tackling the infodemic in Ireland through a comparative analysis of hospitalised cases of COVID-19 and Influenza. Public Health. 2021;194:19–24.
Thompson MG, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe Disease among adults in New Zealand 2012–2015. Vaccine. 2018;36(39):5916–25.
Annual Immunisation. Coverage report 2020. Available from: https://ncirs.org.au/sites/default/files/2022-11/NCIRS%20Annual%20Immunisation%20Coverage%20Report%20SUMMARY%20FINAL.pdf.
De Oliveira Bernardo C, et al. Influenza immunisation coverage from 2015 to 2017: a national study of adult patients from Australian general practice. Vaccine. 2019;37(31):4268–74.
Tin Tin Htar M, et al. The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e48.
Kwon YS, et al. Risk of mortality associated with respiratory syncytial virus and Influenza Infection in adults. BMC Infect Dis. 2017;17(1):785.
Meyer Sauteur PM, et al. Mycoplasma pneumoniae beyond the COVID-19 pandemic: where is it? Lancet Microbe. 2022;3(12):e897.
Greer RM, et al. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract Infections? J Clin Virol. 2009;45(1):10–5.
Pinky L, Dobrovolny HM. Coinfections of the respiratory tract: viral competition for resources. PLoS ONE. 2016;11(5): e0155589.
Schultz-Cherry S. Viral interference: the case of Influenza viruses. J Infect Dis. 2015;212(11):1690–1.
Wan WY, et al. Trends in Respiratory Virus Infections during the COVID-19 pandemic in Singapore, 2020. JAMA Netw Open. 2021;4(6):e2115973-2115973.
Yum S, et al. Trends in viral Respiratory Infections during COVID-19 pandemic, South Korea. Emerg Infect Dis. 2021;27(6):1685–8.
Kuo S-C, et al. Collateral benefit of COVID-19 Control measures on Influenza Activity, Taiwan. Emerg Infect Dis J. 2020;26(8):1928.
Acknowledgements
Not applicable.
Funding
No funding available.
Author information
Authors and Affiliations
Contributions
AG- Writing-original draft. CF- Conceptualizations, writing – review. EP- data curation, formal analysis, validation. AA- Writing review and editing, supervision. CY- Conceptualizations, writing – review, supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been approved by Human Research Ethics Committee, Monash Medical Centre, Monash Health (246 Clayton Road, Clayton Victoria 3168 Australia).
Informed consent waiver approved by the Institutional Review Board of Monash Health (reference number: RES-19-0000-519Q). Given this study was a retrospective extraction of non-identifiable data, consent from individual patients was not required. All experiments were performed in accordance with Institutional Review Board of Monash Health (246 Clayton Road, Clayton Victoria 3168 Australia) guidelines and regulations as set out by the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Grech, A.K., Foo, C.T., Paul, E. et al. Epidemiological trends of respiratory tract pathogens detected via mPCR in Australian adult patients before COVID-19. BMC Infect Dis 24, 38 (2024). https://doi.org/10.1186/s12879-023-08750-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08750-7