Abstract
Background
HIV has become a manageable chronic condition due to the success and scale-up of antiretroviral therapy (ART). Globally, South Africa has the highest number of people living with HIV (PLHIV) and research evidence indicates that countries with the highest burden of PLHIV have a substantial burden of obesity, hypertension (HPT) and type 2 diabetes (T2D). We sought to summarize the burden of these three common NCDs among PLHIV in South Africa.
Methods
In this systematic review, multiple databases were searched for articles reporting on the prevalence of obesity, HPT, and T2D among PLHIV in South Africa published since journal inception until March 2022. A meta-analysis was conducted using random-effects models to obtain pooled prevalence estimates of the three NCDs. Heterogeneity was assessed using X2 test on Cochran’s Q statistic.
Results
We included 32 studies, with 19, 22 and 18 studies reporting the prevalence of obesity, HPT, and T2D among PLHIV, respectively. The overall prevalence of obesity, HPT, and T2D was 23.2% [95% CI 17.6; 29.9], 25.5% [95% CI 15.6; 38.7], and 6.1% [95% CI 3.8; 9.7] respectively. The prevalence of obesity was significantly higher among women (P = 0.034) compared to men, however the prevalence of HPT and T2D did not differ by sex. The prevalence of each of the three NCDs did not differ significantly between rural, urban, and peri-urban areas. The prevalence of obesity and T2D was higher in studies conducted between 2013 and 2022 compared to studies conducted between 2000 and 2012, while the prevalence of HPT was higher between 2000 and 2012 compared to between 2013 and 2022.
Conclusions
These findings suggest that South Africa is experiencing a syndemic of NCDs among people PLHIV highlighting the need to increase cost-effective interventions and management strategies that involve integrated HIV and NCD care in the South African setting.
Similar content being viewed by others
Introduction
Human Immunodeficiency Virus (HIV) is a global public health threat with approximately 40 million people living with HIV (PLHIV). Sub-Saharan Africa (SSA) is the most affected region and South Africa has the highest number of PLHIV (approximately 7.7 million) [1]. Currently HIV has become a manageable chronic condition due to the success and scale-up of antiretroviral therapy (ART) and South Africa has been reported to have the largest ART program globally, with approximately 4.6 million people on treatment [2,3,4,5,6]. Increased life expectancy among PLHIV has become associated with the concurrent increase in the prevalence of non-communicable diseases (NCDs) such as obesity, hypertension (HPT) and type 2 diabetes (T2D) [7, 8]. There is a co-existence of both chronic infectious and non-infectious diseases, both exacerbated by a highly unequal society, poverty and other social determinants, and which reflect an epidemiological transition [1, 9].
The prevalence of obesity is increasing among adults in SSA, with South Africa recording the highest obesity rates, especially among women [10, 11]. Notably, the prevalence of overweight and obesity in South Africa is 67.5% among women and 31.3% among men who are 15 years and above according to the 2016 SA demographic health survey (SADHS) [12]. This has been accompanied by dramatic increases in the prevalence of HPT and T2D [13, 14]. By way of illustration, from 1998 to 2008, the age-adjusted prevalence of HPT increased by more than 10% (rising from 24.4 to 35.1% among both men and women aged 15 years and above) [15]. Furthermore, according to the 2016 SADHS data, the HPT prevalence in women was 46.0% and 44.0% in men [12]. The projected rates of T2D increase (150%) are highest in SSA compared to other International Diabetes Federation (IDF) regions [16], significantly increasing the burden of NCDs in the region. Further, South Africa has the highest number of people with T2D [17] and T2D is the second leading cause of death in the country and the leading cause of death among women [18]. The IDF projects that over 8 million South African adults will be living with T2D by 2045 [16].
Due to the already high prevalence of HIV, obesity, HPT and T2D in the general population of South Africa, there is a need to understand the extent to which the prevalence of these three common NCDs may differ among PLHIV within the South African context. Additionally, because studies comparing rural and urban areas of South Africa have reported different burdens of obesity, HPT and T2D in the general population [19,20,21], it was important to determine if these same patterns in prevalence of NCDs are seen in PLHIV. The juxtaposition of chronic infectious and NCDs in such a context is particularly relevant and could highlight the need for scaling of the South African national government’s plan for integrated chronic disease management.
Therefore, we performed a systematic review and meta-analysis to explore the prevalence of obesity, HPT and T2D among PLHIV in South Africa, in an era of increased life-expectancy among PLHIV. A sub aim was to explore differences in the prevalence by setting (i.e., urban, rural, and peri-urban areas), sex and by year of the study.
Methods
Study design
The systematic review and meta-analysis were guided and reported in accordance with the MOOSE guidelines [22]. We searched for articles in Scopus, Cochrane library, PubMed, Google Scholar, and Web of Science that reported the prevalence of obesity, HPT, and T2D among PLHIV in South Africa published since journal inception until March 2022.
Eligibility criteria
Literature that used objectively measured prevalence estimates of either obesity, HPT or high blood pressure, or T2D among PLHIV within South Africa were included. Studies that used self-report for HPT and T2D were included as well. Peer reviewed journals were not excluded by year of publication or language, but studies must have used human participants. Studies were included irrespective of the study design used (i.e., randomized control trials (RCTs) and observational studies). Papers were excluded if they were qualitative studies, modelling studies, involved pregnant and postpartum women, children, and conducted outside South Africa.
Data collection procedures
Search strategy
A search strategy that employed medical subject headings (MeSH) and keywords was developed and used while searching for literature from all the selected databases. The following key terms were used in the search strategy for all the databases; “South Africa”, “HIV”, “AIDs”, “Epidemiology”, “Prevalence”, “HPT”, “high blood pressure”, “T2D”, “diabetes”, “obese”, and “obesity”. The Boolean terms (AND, OR) were used to separate keywords, and MeSH was conducted in advanced searching of articles. The final search strategy for PubMed can be found in Supplementary Table 1. The same approach was adopted for other databases. A secondary search of relevant articles from reference lists of included studies was also undertaken.
Selection of sources of evidence
Retrieved articles were uploaded into EndNote to remove duplicates. The articles were then uploaded to Rayyan QCRI (Copenhagen: The Nordic Cochrane Centre, Cochrane) [23] where they were independently screened based on title and abstract by two reviewers (JMG, CA). The screening process was done following the eligibility criteria. Then full texts of studies that passed the initial stage of screening were retrieved and screened to verify their conformance with the inclusion criteria. Disagreements on selected studies were discussed and resolved by consensus or the intervention of a third reviewer when necessary.
Statistical methods
Data extraction process
For all included studies, three reviewers (JMG, CA and JD) extracted the data using a data abstraction tool which the authors designed purposely for this study. The following information was extracted: Name of first author and year of publication, study objective, study location (province, rural/urban, site setting), study population (sample size, age), study design, data collection period, NCD studied, diagnostic method, ART status (initiated vs uninitiated), NCD prevalence estimates or data that could be used to self-calculate the prevalence of NCDs among PLHIV. Any disagreements were resolved through discussion between the two reviewers or further adjudication by a third reviewer.
Risk of Bias and quality of included studies
Two reviewers evaluated the methodological quality and risk of bias of included studies using the Risk of Bias tool for prevalence studies developed by Hoy et al. [24].
Synthesizing the results
Data were analyzed with R software (version 3.6.1) using the meta package. Forest plots were drawn to visualize the pooled prevalence and the 95% confidence intervals (CI) of obesity, HPT, and T2D among PLHIV in South Africa and the extent of heterogeneity between studies. Results from pregnant women were excluded while estimating the pooled prevalence of each NCD. A random effects meta-analysis model based on the DErSimonian-Laird inverse-variance method and the Freeman-Tukey double arc-sine transformation for proportions was used to pool the prevalence data because of the inherent differences between the participants in each study [25]. The robustness of the pooled crude prevalence estimate was assessed by doing sensitivity analysis including only studies that had a low risk of bias.
Heterogeneity was assessed using the χ2 test on Cochrane’s Q statistic and quantified by calculating the I2 (with values of 25%, 50% and 75% being representative of low, medium, and high heterogeneity, respectively). Because of the high degree of heterogeneity, subgroup analyses were conducted using the study setting (rural, urban, and peri urban), year in which the study was conducted, sex, diagnostic criteria, and ART status. To assess publication bias, a funnel plot and Egger weighted regression methods were used and a p-value < 0.05 was considered indicative of statistically significant publication bias. Agreement between reviewers for study inclusion, data extraction and risk of bias assessment was assessed using the Cohen’s Kappa coefficient.
Results
Selection of sources of evidence
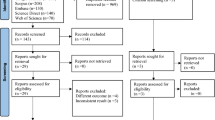
We found a total of 1,558 articles after the search. After removing 280 duplicates, we screened the titles and abstracts of 1,278 articles and excluded 1,211 that did not meet our inclusion criteria. We then reviewed the full texts of 65 articles and excluded 33 articles for the following reasons: 7 articles involved pregnant or postpartum women; 17 articles had no results on outcomes of interest; 1 article was not done in South Africa; 3 articles did not report results for HIV participants; 1 article was a duplicate but with a different title; 2 articles were systematic reviews because relevant articles contained in these reviews had already been selected for inclusion; and 2 articles were modelling studies. The full text of two articles were not accessible. In total, 32 studies that reported prevalence estimates for obesity, HPT and T2D among PLHIV in South Africa were reviewed (Fig. 1).
Characteristics of sources of evidence
The characteristics of the included studies; the province, study setting, study design, sample size, population sample age and sex and the risk of bias for each study are presented in Table 1. The studies were published between 2004 and 2020, although 66.7% of the studies were published after 2012. Of the 32 studies, 22 studies used a cross-sectional design, 6 were cohort studies, and 4 were national and community-based surveys. The majority of the studies (n = 9, 28.1%) were conducted in Western Cape (WC), KwaZulu Natal (KZN, n = 9, 28.1%) and Gauteng (GA, n = 5, 15.6%), with less representation of the Eastern Cape (EC), Free State (FS), Mpumalanga (MP) and Limpopo (LM) provinces. No studies were included from Northern Cape and Northwest provinces. The majority (n = 20, 62.3%) of studies were conducted in health care facility settings and the remainder were community-based studies. In terms of study setting, most of the studies (n = 15, 46.9%) were conducted in urban areas, followed by 6 (18.8%) studies in peri-urban townships and only 5 (15.6%) studies were conducted in a rural area, while 6 (18.8%) studies were conducted both in urban and rural areas. Out of the 32 studies, 5 were conducted among women only, 26 studies included both men and women, and 1 study included only men. Most studies (n = 30) were conducted amongst adults (18 years and older), and 1 study included participants who were 15 years and older, and 1 study did not report the age range of the participants. Of the included studies, 18 (56.3%) involved participants that were on ART, 10 (31.3%) studies involved participants who were not on ART or who initiated ART during the study, 1 study included both ART initiated and uninitiated participants while 3 studies did not report the ART status of included participants. Among the included studies, 19 (59.4%), 22 (68.8%), and 18 (56.3%) reported prevalence rates for obesity, HPT, and T2D, respectively. Twelve studies exclusively examined one of the NCDs, 14 studies investigated two NCDs, and 6 studies investigated all three NCDs simultaneously.
Prevalence of obesity among PLHIV in South Africa
Overall, data from 13,779 PLHIV from 19 studies that reported prevalence of obesity were included. All 19 studies used the WHO criteria as reference for body mass index (BMI) cut-off values for obesity [57]. Most of the studies that reported the prevalence of obesity were conducted in urban (n = 11) settings followed by rural (n = 4), peri-urban (n = 3) settings and one study was conducted both in urban and rural settings. Meta-analysis data indicate that the overall prevalence of obesity in South Africa among PLHIV was 23.2% [95% CI 17.6; 29.9] with high heterogeneity (I2 = 99.0%, p < 0.001) (Table 2, Fig. 2). Based on the funnel plot (Supplementary Fig. 1) and Egger’s test, there was symmetry and no evidence of potential publication bias (p = 0.202) for determining the prevalence of obesity among PLHIV. The sensitivity analysis including only low risk of bias studies yielded a slightly lower prevalence estimate to that of the crude analysis (Table 2).
The prevalence of obesity was significantly higher (p = 0.034) in women (23.6% [95% CI 17.4; 31.2]) compared to men (12.1% [95% CI 6.3; 22.1]) (Table 2, Fig. 3). The pooled prevalence was higher in studies conducted in peri-urban (25.4% [95% CI 10.3; 50.3]) and urban (25.0% [95% CI 17.3; 34.8]) compared to rural areas (21.5% [95% CI 5.6; 55.6]) (Table 2, Fig. 4). The prevalence was higher in studies conducted between 2013–2022 (25.8% [95% CI 18.8; 34.2]) compared to studies conducted between 2000–2012 (19.7 [95% CI 10.8; 33.3]) (Fig. 2). The prevalence was also higher in studies conducted among participants who were on ART (25.9% [95% CI 19.2; 33.9]) compared to studies conducted among participants not initiated on ART (22.7 [95% CI 11.1; 40.7]) (Table 2).
Prevalence of HPT among PLHIV in South Africa
In total, data from 123,951 PLHIV from 22 studies that reported prevalence of HPT were included. Different diagnostic criteria were utilised to categorise participants as hypertensive or normotensive. These included: participant self‐reported history of HPT diagnosis by a professional physician (n = 1); self-reported use of antihypertensive drugs (n = 1), blood pressure (BP) measurements (n = 11) whereby a participant with systolic BP readings of ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg was considered hypertensive; either BP measurement or self-reported use of antihypertensive drugs (n = 8); either BP measurement or self-reported history of hypertension (n = 1).
Overall, the prevalence of HPT among PLHIV in South Africa was 25.5% [95% CI 15.6; 38.7], and the heterogeneity between the studies was high (I2 = 99.0%, p < 0.001) (Table 3, Fig. 5). The Egger’s test and the funnel plot suggested no evidence of publication bias (p = 0.937) (Supplementary Fig. 2). The sensitivity analysis including only low risk of bias studies yielded a similar prevalence estimate to that of the crude analysis (Table 3).
The pooled prevalence of HPT among men and women was 22.4% [95% CI 11.7;38.6] and 27.0% [95% CI 14.8;44.0] respectively (Table 3, Fig. 6). The pooled prevalence for studies conducted in rural areas was 25.6% [95% CI 7.4; 59.9]), peri-urban areas 38.0% [95% CI 8.6; 80.0] and urban areas 18.5% [95% CI 4.3;53.7]) (Table 3, Fig. 7). The prevalence was lower in studies conducted among participants who were on ART (24.2% [95% CI 11.1; 44.9]) compared to studies conducted among participants not initiated on ART (34.1 [95% CI 9.8; 71.1]) (Table 3).
The pooled prevalence was higher in studies conducted between 2000–2012 (34.1% [95% CI 15.2;59.9]) compared to studies conducted between 2013–2022 (20.0% [95% CI 9.8;36.5]) (Table 3, Fig. 5). Based on diagnostic criteria, studies that used BP only measurement had a pooled prevalence of 26.7% [95% CI 11.5;50.5], while in studies those that used BP measurement or antihypertensive drug use, the prevalence was 20.7% [95% CI 12.2;32.9], and the two studies that used the self-report criteria yielded a pooled prevalence of 45.8% [95% CI 0.0;100] (Table 3).
Prevalence of T2D among PLHIV in South Africa
In total, data from 18,555 PLHIV were included from the 18 studies that reported on prevalence of T2D. The diagnostic criteria used to categorise participants as having diabetes varied widely across the studies and included: participant self‐reported history of diagnosed T2D by a professional physician (n = 1); self-reported use of insulin or oral hypoglycemic medications (n = 1); fasting blood glucose (FBG) ≥ 7.0 mmol/L (n = 1); a random blood glucose level ≥ 11.1 mmol/L (n = 1); oral glucose tolerance test (OGTT) ≥ 200mg/dL (n = 2); HbA1c ≥ 6.5% (n = 5); either self-reported use of insulin or FBG (n = 2); either self-report or OGTT or FBG or HbA1c (n = 1); either non FBG or FBG or medication use (n = 1); either medication use or HbA1c ≥ 6.5% (n = 1). Two studies did not explicitly report the diagnostic criteria used. Most of the studies that reported prevalence of T2D were conducted in an urban setting (n = 9).
The overall prevalence of T2D among PLHIV in South Africa was 6.1% [95% CI 3.8; 9.7] with high heterogeneity (I2 = 99.0%, p < 0.001) (Table 4, Fig. 8). The funnel plot (Supplementary Fig. 3) suggested publication bias confirmed by the Egger’s regression test (p = 0.011). The sensitivity analysis including only low risk of bias studies yielded a prevalence estimate of 8.0% [95% CI 4,5;13.8] (Table 4). In the subgroup analysis, the pooled prevalence was higher in studies conducted in rural areas (14.0% [95% CI 0.7;79.6]), compared to peri-urban and urban areas (Table 4, Fig. 9). However, the prevalence did not differ significantly by study area. When studies were grouped based on the period when they were conducted, the prevalence was higher in studies conducted between 2013 and 2022 (8.1% [95% CI 4.1; 15.2]) compared to studies conducted between 2000 and 2012 (4.4% [95% CI 2.3; 8.4]) (Table 4, Fig. 8). There was no difference in T2D prevalence between men and women and based on ART status (Table 4, Fig. 10). Further, the pooled prevalence didn’t differ based on the diagnostic criteria.
Meta-analysis of prevalence of Type 2 diabetes among people living with HIV in South Africa grouped by setting (rural, peri-urban, and urban areas) CI: confidence interval. The vertical dotted line represents the overall prevalence, and the red diagonals represent the pooled prevalence in each setting
Discussion
This systematic review and meta-analysis focused on the syndemics of obesity, HPT and/or T2D with HIV, describing the prevalence of these three NCDs in PLHIV in South Africa. Overall, the prevalence of HPT was highest among PLHIV followed closely by obesity and then T2D. Obesity was higher in PLHIV from urban and peri-urban areas compared to rural areas, while the prevalence of HPT and T2D were higher among PLHIV in rural and peri-urban areas compared to urban areas. The prevalence of obesity and T2D have both increased over time while the prevalence of HPT has decreased over time. Generally, our findings represent pooled estimates of the burden of obesity, HPT, and T2D in PLHIV in South Africa factoring in differences in study setting, period when the studies were conducted, ART status, sex of the participants and diagnostic criteria.
Obesity
The pooled prevalence of obesity in 13,779 PLHIV from 19 studies was 23.2% with women having a higher prevalence (23.6%) compared to men (12.1%). Our findings are lower than the prevalence estimates that have been reported in the general population of South Africa women (41%) but similar to the men (11%) [58]. Furthermore, our findings agree with the obesity prevalence estimates that have been reported among men and women living with HIV in other low middle income countries (LMIC) in SSA such as in Kenya (women: 22.6%, men: 11.0%) and Nigeria [59,60,61]. The fact that obesity is still prevalent among PLHIV may be explained by the success of widespread use of ART in South Africa with approximately 4.6 million adults on ART [5]. This is partially supported by the finding reported in this review indicating that participants initiated on ART had a higher prevalence of obesity compared to participants not initiated on ART. ART is reported to be associated with weight gain among PLHIV and evidence of the dual occurrence of obesity and HIV epidemics is increasing in the literature [62,63,64,65,66]. For instance, dolutegravir (DTG) has been reported to impact weight and glucose metabolism among PLHIV [67, 68]. Further, the high burden of obesity in this review is suggestive of co-existence of chronic NCDs with chronic infectious diseases and may reflect an epidemiological transition in the South African population. Moreover, elevated rates of obesity are indicative of increased risk of Cardiovascular diseases, T2D, and musculoskeletal disorders [69].
Our findings indicate that the prevalence of obesity among PLHIV did not differ significantly based on study area, with estimates being relatively similar in urban, peri-urban, and rural areas. This could be attributed to the overall changes in lifestyle and nutritional transition, which are associated with increased access to energy-dense foods, animal-source foods, sugar-sweetened beverages, and less strenuous jobs [66, 70, 71]. However, shifts in the diet are more evident among people residing in urban settings compared to those in rural settings because of higher income and easy access to processed foods [11, 71]. Furthermore, our combined estimates indicated that the prevalence in studies conducted from 2013 to 2022 was lower than that in studies conducted between 2000 and 2012. These findings contradict the reported increase in prevalence in South Africa and SSA as a whole [11]. It's important to note that our results lacked statistical significance and may have been influenced by the substantial heterogeneity in the included studies.
Hypertension
In this meta-analysis, 22 studies with 123,951 PLHIV reported on the prevalence of HPT, and the pooled prevalence was 25.5%. When analyzed by sex, the prevalence estimates among women (27.0%) and men (22.4%) living with HIV were lower than that reported in the 2016 Demographic Health Survey (DHS) where the HPT prevalence was 46.0% among women and 44.0% among men in the general population of South Africa [58]. Variations could be explained by the diverse diagnostic criteria that were used by the studies included in this review, that involved both objective measurements and participant recall. The 2016 DHS classified HPT using objective measurements (Blood pressure WHO cutoff values). HPT is significantly underdiagnosed in the general population of South African [72, 73] and among PLHIV [29]. Further, limited integration of HIV programs with NCD care during the period when most included studies were conducted could be a barrier to precise ascertainment of HPT burden among PLHIV in South Africa.
In other studies, conducted globally and in SSA, the prevalence of HPT has been shown to be high among PLHIV compared to people not infected with HIV [74, 75]. The most recent meta-analysis on the global estimates of HPT prevalence among PLHIV involving 194 studies from 61 countries with data collected between 2007 and 2018 reported a global pooled prevalence of 23.6% [95% CI 21.6%; 25.5%] and 19.9% [95% CI 17.2; 22.8%] in the Southern and Eastern region of Africa [76]. Our findings correspond to the estimates in SSA and in the Southern African region. In a systematic review by Nguyen et al., [77], the prevalence of HPT was reported to vary widely (ranging from 8.7% to 45.9%) among PLHIV in LMICs, concluding that the burden of HPT among PHLIV is not well established in LMIC. Future research which allows for accurate estimates of diagnosed HPT prevalence among PLHIV in South Africa are necessary to guide health policies for effective management of the dual burden of HIV and HPT.
Our finding showed that the prevalence of HPT among PLHIV did not differ significantly based on area, however rural and peri-urban areas had a higher prevalence compared to urban areas. Similar findings have been reported in studies conducted to estimate burden of HPT in the general South Africa population [19, 78]. In contrast Addo et al., [79], reported the majority of hypertensive patients to be urban dwellers in SSA. Based on our findings, there is a need to develop and intensify existing healthcare systems in rural areas and peri-urban townships without overlooking urban areas to optimize HPT care especially among PLHIV.
Type 2 diabetes
The overall prevalence of T2D in 18 studies with 18,555 PLHIV was 6.1% with high heterogeneity between the individual studies. Stratified by sex, our findings showed that the prevalence was similar in both men and women (6.7% and 6.2% respectively).Studies such as a meta-analysis by Pheiffer et al.,[21], a survey study by Egede et al. [80], and a study by Grundlingh et al. [81], reported varying prevalence estimates of 15.3%, 1.7% and 22% respectively in the general South African population. The difference in the findings may be attributed to wide diversity of study populations, differences in the participants’ lifestyles and a wide variety of diagnostic criteria used. For instance, in this review, different criteria were used to diagnose T2D such as FBG, OGTT, HbA1c, non FBG, insulin use and self-report, hence resulting in varying estimates of T2D burden. OGTT is the recommended criterion for diagnosis of T2D in Africa [82]. Standardisation of the diagnostic criteria will help address issue of variation in diagnoses. Our reported overall prevalence estimates contrast to studies conducted in different parts of SSA [83,84,85]. Variations in estimates may be partly attributed to challenges with proper diagnosis of T2D in PLHIV in South Africa, whereby a significant proportion of patients are underdiagnosed [81, 86]. With the increasing T2D related risks such as rapid urbanisation, physical inactivity, and unhealthy diets in South Africa, continued monitoring and tracking of T2D at both population and individual levels mostly among PLHIV is necessary.
Our findings show that the pooled prevalence of T2D among PLHIV was higher in rural (14.0%), and peri-urban (8.6%) settings compared to urban settings (4.3%). Though unsignificant, these findings are contrary to other results reported in South Africa and SSA where the prevalence was reported to be higher in urban settings [20, 87]. Because of the existing association between HIV infection and increased risk of T2D [88, 89], limited access to healthcare, and high unemployment in both urban and rural settings of South Africa, there is need for integration of screening, monitoring and interventions for T2D into HIV programs.
The pooled prevalence of T2D was higher in studies conducted between 2013 and 2022 compared to 2000 and 2012. The results, which demonstrate an increase in T2D in more recent times, agree with those reported in previous studies, systematic reviews, and meta-analyses [74, 76, 89]. This could be explained by increased life expectancy among PLHIV [90] and epidemiological transitions in South Africa such as population growth and aging [91, 92]. In addition, ART coverage has improved in South Africa and globally resulting in longer life and reduced mortality rates among PLHIV [6, 90]. Also, the national ART treatment guidelines have been changing ART roll-out in SA, for instance in 2009, ART guidelines moved from monotherapy to dual therapy [93], this may be a salient factor influencing the observed trends in co-morbidities between the older papers and more recent ones. Furthermore, the elevated estimates of T2D could also be due to an increasing burden of obesity, increasing urbanization, adaptation of lifestyle behaviors and their interaction with the genetic predisposition to T2D within the South African population [94, 95].
Implication of the findings
The findings in this meta-analysis have implications on practice and further research of syndemics of NCDs and HIV in South Africa and other LMICs with high burden of HIV. The high and varying prevalence of obesity, HPT and T2D in PLHIV highlights the need for scaling of the South Africa national government’s plan for integrated chronic disease management. Integration of NCD services such as screening, diagnosis, prevention (primary and secondary) and treatment into HIV programs has been shown to be beneficial in addressing the increasing dual burden of HIV and cardiovascular diseases risk factors (obesity, T2D, and HPT) [7, 96,97,98]. Due to the high burden of HIV, South African health care system should be strengthened to face the increasing dual burden of NCDs and HIV.
Limitations
This meta-analysis is not without limitations. Firstly, the review included some studies with moderate risk of bias; although after considering only studies with low risk of bias, the overall estimates were similar to the crude analysis estimates. Secondly, there was high heterogeneity between the studies, an issue common to meta-analysis of prevalence studies, therefore the pooled estimates need to be interpreted with caution. Higher heterogeneity increases uncertainty in the pooled estimates and reduces generalizability of the results. Sub-group analyses, based on settings (rural, urban, peri-urban), sex, ART status, diagnostic criteria, and study year, were conducted to pinpoint sources of heterogeneity, though the issue persisted. Notably, some other variables that could have contributed to the observed heterogeneity in the original studies such as CD4 cell count, HIV viral load, duration on ART, ART regimen and sociodemographic factors were not explicitly reported. Additionally, some subgroup analyses were not statistically significant hence it’s not definitive that the prevalence estimate for one subgroup is higher or lower compared to another. Thirdly, caution is needed when using obesity estimates, as many studies relied solely on BMI, which may not be suitable due to factors such as genetic diversity and sociocultural factors in Sub-Saharan Africa. Complementary assessments like waist circumference, body composition, and metabolic risk factors offer a more comprehensive assessment. Finally, the provinces were variably represented with more studies in WC, KN and GA provinces compared to other provinces in South Africa. Hence weakening generalizability of our findings and requiring that more studies on NCDs among PLHIV are conducted in NC, EC, LM, FS, NW and MP provinces. Irrespective of these limitations, this meta-analysis provided a clear summary of the existing evidence on the burden of obesity, HPT and T2D among PLHIV in South Africa according to setting (rural, peri-urban, or urban area), sex, and how the burden has varied over time in the era of increased life expectancy among PLHIV.
Conclusion
We found that among PLHIV in South Africa, the prevalence of HPT was highest, closely followed by obesity, and then T2D. The pooled prevalence estimates for obesity and HPT were higher in peri-urban areas, whereas the pooled prevalence of T2D was higher in rural areas. The prevalence estimates of obesity and T2D have increased over time. These findings add to the already existing literature about the increasing burden of cardiovascular disease risk factors, specifically obesity, HPT and T2D in LMIC with a high burden of HIV. However, caution needs to be taken while interpreting these results because of heterogeneity in the in studies. More research focusing on understanding syndemics of NCDs, and HIV epidemics is needed particularly in setting with a high burden of HIV. This would then guide enhancement of cost-effective interventions that involve integrated HIV and NCDs care such as screening, diagnosis, prevention, and treatment programs in LMIC faced with a dual burden of HIV and NCD epidemics.
Availability of data and materials
All data analyzed during this study are included in this published article [and its supplementary information files].
References
UNAIDS, "Global AIDS Update," 2016.
Heestermans T, Browne JL, Aitken SC, Vervoort SC, Klipstein-Grobusch K. Determinants of adherence to antiretroviral therapy among HIV-positive adults in sub-Saharan Africa: a systematic review. BMJ Glob Health. 2016;1(4):e000125. https://doi.org/10.1136/bmjgh-2016-000125.
Iacob SA, Iacob DG, Jugulete G. “Improving the Adherence to Antiretroviral Therapy, a Difficult but Essential Task for a Successful HIV Treatment-Clinical Points of View and Practical Considerations,” (in eng). Front Pharmacol. 2017;8:831–831. https://doi.org/10.3389/fphar.2017.00831.
Masquillier C, et al. Households in HIV Care: Designing an Intervention to Stimulate HIV Competency in Households in South Africa. Front Public Health. 2020;8:246. https://doi.org/10.3389/fpubh.2020.00246.
UNAIDS, "UNAIDS Data 2019," 2019.
Phillips TK, Myer L. Shifting to the long view: engagement of pregnant and postpartum women living with HIV in lifelong antiretroviral therapy services. Expert Rev Anti Infect Ther. 2019;17(5):349–61. https://doi.org/10.1080/14787210.2019.1607296.
Patel P, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis,. AIDS. 2018;32(Suppl 1):S5-s20. https://doi.org/10.1097/qad.0000000000001888.
van Heerden A, Barnabas S, Norris A, Micklesfield LK, van Rooyen H. High prevalence of HIV and non-communicable disease (NCD) risk factors in rural KwaZulu-Natal, South Africa. J Int AIDS Soc. 2017;20:e25012.
Levitt NS, Steyn K, Dave J, Bradshaw D. Chronic noncommunicable diseases and HIV-AIDS on a collision course: relevance for health care delivery, particularly in low-resource settings—insights from South Africa. Am J Clin Nutr. 2011;94(6):1690S-1696S.
Puoane T, et al. Obesity in South Africa: The South African Demographic and Health Survey. Obes Res. 2002;10(10):1038–48. https://doi.org/10.1038/oby.2002.141.
Ajayi IO, et al. “Urban-rural and geographic differences in overweight and obesity in four sub-Saharan African adult populations: a multi-country cross-sectional study,” (in eng). BMC Public Health. 2016;16(1):1126–1126. https://doi.org/10.1186/s12889-016-3789-z.
(2019). South Africa demographic and health survey 2016.
Oladeji O, et al. “Monitoring Information-Seeking Patterns and Obesity Prevalence in Africa With Internet Search Data: Observational Study,” (in eng). JMIR Public Health Surveill. 2021;7(4):e24348–e24348. https://doi.org/10.2196/24348.
Patel SA, et al. “Obesity and its Relation With Diabetes and Hypertension: A Cross-Sectional Study Across 4 Geographical Regions,” (in eng). Glob Heart. 2016;11(1):71-79.e4. https://doi.org/10.1016/j.gheart.2016.01.003.
Berry KM, et al. “Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011–2012,” (in eng). BMJ Glob Health. 2017;2(3):e000348–e000348. https://doi.org/10.1136/bmjgh-2017-000348.
Saeedi P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843.
Motala A A, Mbanya JC, Ramaiya K, Pirie FJ, Ekoru K. Type 2 diabetes mellitus in sub-Saharan Africa: challenges and opportunities. Nat Rev Endocrinol. 2022;18:219–29. https://doi.org/10.1038/s41574-021-00613-y.
S. S. Africa, "Mortality and causes of death in South Africa: Findings from death notification 2018," 2018.
Ntuli ST, Maimela E, Alberts M, Choma S, Dikotope S. Prevalence and associated risk factors of hypertension amongst adults in a rural community of Limpopo Province, South Africa. Afr J Prim Health Care Fam Me. 2015;7(1):847. https://doi.org/10.4102/phcfm.v7i1.847.
Peltzer K, Phaswana-Mafuya N, Pengpid S. “Rural-urban health disparities among older adults in South Africa,” (in eng). Afr J Prim Health Care Family Med. 2019;11(1):e1–6. https://doi.org/10.4102/phcfm.v11i1.1890.
Pheiffer C, Pillay-van Wyk V, Turawa E, Levitt N, Kengne AP, Bradshaw D. Prevalence of Type 2 Diabetes in South Africa: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2021;18(11):5868. https://doi.org/10.3390/ijerph18115868.
Stroup DF, et al. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA. 2000;283(15):2008–12. https://doi.org/10.1001/jama.283.15.2008.
A. Elmagarmid, Z. Fedorowicz, H. Hammady, I. Ilyas, M. Khabsa, and M. Ouzzani, "Rayyan: a systematic reviews web app for exploring and filtering searches for eligible studies for Cochrane Reviews," in Evidence-Informed Public Health: Opportunities and Challenges. Abstracts of the 22nd Cochrane Colloquium, 2014: John Wiley & Sons Hyderabad, India, India, pp. 21–26.
Hoy D, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–9. https://doi.org/10.1016/j.jclinepi.2011.11.014.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials. 2007;28(2):105–14. https://doi.org/10.1016/j.cct.2006.04.004.
Z. Abrahams, J. A. Dave, G. Maartens, and N. S. Levitt, "Changes in blood pressure, glucose levels, insulin secretion and anthropometry after long term exposure to antiretroviral therapy in South African women," (in English), AIDS Research and Therapy, vol. 12, no. 1, 2015 2015. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84938562170&doi=10.1186%2fs12981-015-0065-8&partnerID=40&md5=5d33b016f90f60228e463e8e28987c3c.
M. S. Borkum et al., "High prevalence of “non-dipping” blood pressure and vascular stiffness in HIV-infected South Africans on antiretrovirals," (in English), PLoS ONE, vol. 12, no. 9, 2017 2017. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85031707262&doi=10.1371%2fjournal.pone.0185003&partnerID=40&md5=f4918000ae304041c4f19655f944553f.
Gausi B, Berkowitz N, Jacob N, Oni T. Treatment outcomes among adults with HIV/non-communicable disease multimorbidity attending integrated care clubs in Cape Town, South Africa. AIDS Res Ther. 2021;18(1):72.
George S, McGrath N, Oni T. The association between a detectable HIV viral load and non-communicable diseases comorbidity in HIV positive adults on antiretroviral therapy in Western Cape, South Africa. BMC Infect Dis. 2019;19:348. https://doi.org/10.1186/s12879-019-3956-9.
Hyle EP, et al. Cardiovascular risk factors among ART-experienced people with HIV in South Africa. J Int AIDS Soc. 2019;22(4):e25274.
Levitt NS, et al. Increased risk of dysglycaemia in South Africans with HIV; especially those on protease inhibitors. Diabetes Res Clin Pract. 2016;119:41–7.
Mutemwa M, et al. Prevalence, detection, treatment, and control of hypertension in human immunodeficiency virus (HIV)-infected patients attending HIV clinics in the Western Cape Province, South Africa. Medicine. 2018;97(35):e12121.
T. Oni, N. Berkowitz, M. Kubjane, R. Goliath, N. S. Levitt, and R. J. Wilkinson, "Trilateral overlap of tuberculosis, diabetes and HIV-1 in a high-burden African setting: implications for TB control," EUROPEAN RESPIRATORY JOURNAL, vol. 50, no. 1, JUL-1 2017.
T. Oni, E. Youngblood, A. Boulle, N. McGrath, R. J. Wilkinson, and N. S. Levitt, "Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study," BMC INFECTIOUS DISEASES, vol. 15, JAN-17 2015.
T. Barnighausen et al., "Hiding in the shadows of the HIV epidemic: obesity and hypertension in a rural population with very high HIV prevalence in South Africa," JOURNAL OF HUMAN HYPERTENSION, vol. 22, no. 3, pp. 236–239, MAR 2008.
C. Biggs and E. Spooner, "Obesity and HIV: a compounding problem," SOUTH AFRICAN JOURNAL OF CLINICAL NUTRITION, vol. 31, no. 4, pp. 78–83, 2018 2018.
K. Naidoo, N. Yende-Zuma, and S. Augustine, "A retrospective cohort study of body mass index and survival in HIV infected patients with and without TB co-infection," (in English), Infect. Dis. Pover., vol. 7, no. 1, 2018 2018. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85045933010&doi=10.1186%2fs40249-018-0418-3&partnerID=40&md5=b5ca00dd65a4f8a015770d4b7b7734d6.
S. Hanley, D. Moodley, and M. Naidoo, "Obesity in young South African women living with HIV: A cross-sectional analysis of risk factors for cardiovascular disease," (in English), PLoS ONE, vol. 16, no. 11, 2021 2021. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85119267064&doi=10.1371%2fjournal.pone.0255652&partnerID=40&md5=0ab70c45573c6cd091c79d34169e2ae6.
E. Hurley, A. Coutsoudis, J. Giddy, S. E. Knight, E. Loots, and T. M. Esterhuizen, "Weight evolution and perceptions of adults living with HIV following initiation of antiretroviral therapy in a South African urban setting," (in English), S. Afr. Med. J., vol. 101, no. 9, pp. 645–650, 2011 2011. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-80052563324&partnerID=40&md5=d43f609eeb44debbc190ebe34273e4f8.
A. Malaza, J. Mossong, T. Barnighausen, and M. L. Newell, "Hypertension and Obesity in Adults Living in a High HIV Prevalence Rural Area in South Africa," PLoS ONE, vol. 7, no. 10, OCT-17 2012.
D. M. Umar and P. Naidoo, "Prevalence and predictors of diabetes mellitus among persons living with HIV: a retrospective cohort study conducted in 4 public healthcare facilities in KwaZulu-Natal," BMC Public Health, vol. 21, no. 1, FEB-4 2021.
A. Rajagopaul and M. Naidoo, "Prevalence of diabetes mellitus and hypertension amongst the HIV-positive population at a district hospital in eThekwini, South Africa," AFRICAN JOURNAL OF PRIMARY HEALTH CARE & FAMILY MEDICINE, vol. 13, no. 1, SEP-29 2021.
K. L. Hopkins et al., "Demographics and health profile on precursors of non-communicable diseases in adults testing for HIV in Soweto, South Africa: A cross-sectional study," (in English), BMJ Open, vol. 9, no. 12, 2019 2019. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85076682388&doi=10.1136%2fbmjopen-2019-030701&partnerID=40&md5=f4d6cfa5ea62b9ed9817f52b95241e19.
Hopkins KL, et al. The substantial burden of non-communicable diseases and HIV- comorbidity amongst adults: Screening results from an integrated HIV testing services clinic for adults in Soweto, South Africa. eClinicalMedicine. 2021;38:101015.
Julius H, et al. The Burden of Metabolic Diseases Amongst HIV Positive Patients on HAART Attending the Johannesburg Hospital. Curr HIV Res. 2011;9:247–52.
K. Mahlangu, P. Modjadji, and S. Madiba, "The Nutritional Status of Adult Antiretroviral Therapy Recipients with a Recent HIV Diagnosis; A Cross-Sectional Study in Primary Health Facilities in Gauteng, South Africa," HEALTHCARE, vol. 8, no. 3, SEP 2020.
F. Mashinya, M. Alberts, R. Colebunders, and J. P. Van Geertruyden, "Cardiovascular risk factors in a treatment-naïve, human immunodeficiency virus-infected rural population in Dikgale, South Africa," (in English), South African Family Practice, vol. 56, no. 3, pp. 190–195, 2014 2014. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84906048516&doi=10.1080%2f20786204.2014.936665&partnerID=40&md5=e1aa9d303c88d3b401d9f671ab845e66.
R. L. Mathebula, E. Maimela, and N. S. Ntuli, "The prevalence of selected non-communicable disease risk factors among HIV patients on anti-retroviral therapy in Bushbuckridge sub-district, Mpumalanga province," (in English), BMC Public Health, vol. 20, no. 1, 2020 2020. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85079605693&doi=10.1186%2fs12889-019-8134-x&partnerID=40&md5=f5d4bbc4f7a582e0290d9ce3269bed37.
M. Rabkin, A. Mutiti, C. Chung, Y. Zhang, Y. Wei, and W. M. El-Sadr, "Missed Opportunities to Address Cardiovascular Disease Risk Factors amongst Adults Attending an Urban HIV Clinic in South Africa," PLoS ONE, vol. 10, no. 10, OCT-8 2015.
Z. Hattingh, C. Walsh, and C. J. Bester, "Anthropometric profile of HIV-uninfected and HIV-infected women aged 25–44 years in Mangaung, Free State," (in English), South African Family Practice, vol. 53, no. 5, pp. 474–480, 2011 2011. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84871037318&doi=10.1080%2f20786204.2011.10874137&partnerID=40&md5=a526fb4b8cea5ace9f791096fe40eb96.
O. O. Sogbanmu et al., "Diagnosing diabetes mellitus with glycated haemoglobin in newly diagnosed HIV-positive patients in buffalo city municipality, South Africa: A crosssectional study," (in English), Open Public Health J., vol. 12, no. 1, pp. 263–268, 2019 2019. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85071100808&doi=10.2174%2f1874944501912010263&partnerID=40&md5=4ce7832ec72e8ebb0bac2f3d749587ed.
Wensink GE, Schoffelen AF, Tempelman HA, Rookmaaker MB, Hoepelman AI, Barth RE. Albuminuria Is Associated with Traditional Cardiovascular Risk Factors and Viral Load in HIV-Infected Patients in Rural South Africa. PLoS ONE. 2015;10(8):e0136529.
Zungu NP, et al. Prevalence of non-communicable diseases (NCDs) and associated factors among HIV positive educators: Findings from the 2015/6 survey of Health of Educators in Public Schools in South Africa. PLoS ONE. 2019;14(2):e0209756.
Brennan AT, et al. Prevalence, incidence, predictors, treatment, and control of hypertension among HIV-positive adults on antiretroviral treatment in public sector treatment programs in South Africa. PLoS ONE. 2018;13(10):e0204020.
Chiw, N. ire, N. Zungu, M. Mabaso, and C. Chasela, "Trends, prevalence and factors associated with hypertension and diabetes among South African adults living with HIV, 2005–2017," (in English), BMC Public Health, vol. 21, no. 1, 2021 2021. [Online]. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85102087321&doi=10.1186%2fs12889-021-10502-8&partnerID=40&md5=eb14c95a7d3178f0a3a2ae45e8bd3a7e.
Godongwana M, De Wet-Billings N. Time to hypertension development among people living with HIV in South Africa: A longitudinal analysis of the National Income Dynamics Survey (NIDS). Heliyon. 2021;7(8):e07750.
"Global Status Report on noncommunicable diseases 2014. World Health Organization." World Health Organization. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf (accessed.
H. National Department of and Icf, "South Africa Demographic and Health Survey 2016," National Department of Health - NDoH - ICF, Pretoria, 2019. [Online]. Available: http://dhsprogram.com/pubs/pdf/FR337/FR337.pdf
Bloomfield GS, et al. Hypertension and Obesity as Cardiovascular Risk Factors among HIV Seropositive Patients in Western Kenya. PLoS ONE. 2011;6(7):e22288. https://doi.org/10.1371/journal.pone.0022288.
Ezechi LO, Musa ZA, Otobo VO, Idigbe IE, Ezechi OC. Trends and risk factors for obesity among HIV positive Nigerians on antiretroviral therapy. Ceylon Med J. 2016;61(2):56. https://doi.org/10.4038/cmj.v61i2.8300.
Okeke O, Ogunfowokan O, Moses L, Umoru D. Overweight and obesity among HIV-infected patients in a West African population. Int J Med Biomed Res. 2017;6(2):65–73.
Koethe JR, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016;32(1):50–8.
Gomes A, et al. “Incidence of Diabetes Mellitus and Obesity and the Overlap of Comorbidities in HIV+ Hispanics Initiating Antiretroviral Therapy,” (in eng). PLoS ONE. 2016;11(8):e0160797–e0160797. https://doi.org/10.1371/journal.pone.0160797.
Bakal DR, et al. “Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors,” (in eng). J Antimicrob Chemother. 2018;73(8):2177–85. https://doi.org/10.1093/jac/dky145.
Ezechi L, Musa Z, Otobo V, Idigbe I, Ezechi O. Trends and risk factors for obesity among HIV positive Nigerians on antiretroviral therapy. Ceylon Med J. 2016;61(2):56–62.
Stringhini S, et al. “The social patterning of risk factors for noncommunicable diseases in five countries: evidence from the modeling the epidemiologic transition study (METS),” (in eng). BMC Public Health. 2016;16(1):956–956. https://doi.org/10.1186/s12889-016-3589-5.
Bourgi K, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267–74.
Menard A, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS. 2017;31(10):1499–500.
Steyn NP, Mchiza ZJ. Obesity and the nutrition transition in Sub-Saharan Africa. Ann N Y Acad Sci. 2014;1311(1):88–101.
Sodjinou R, Agueh V, Fayomi B, Delisle H. Obesity and cardio-metabolic risk factors in urban adults of Benin: relationship with socio-economic status, urbanisation, and lifestyle patterns. BMC Public Health. 2008;8(1):1–13.
A. O. Sola, A. O. Steven, J. A. Kayode, and A. O. Olayinka, "Underweight, overweight and obesity in adults Nigerian living in rural and urban communities of Benue State," Annals of African medicine, vol. 10, no. 2, 2011.
Kamerman P. Underdiagnosis of hypertension and diabetes mellitus in South Africa. S Afr Med J. 2022;112(1):13519.
Day C, Groenewald P, Laubscher R, van Schaik N, Bradshaw D. Monitoring of non-communicable diseases such as hypertension in South Africa: Challenges for the post-2015 global development agenda. S Afr Med J. 2014;104(10):680–7.
Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11(8):530–40. https://doi.org/10.1016/j.jash.2017.06.004.
Okello S, et al. Incidence and predictors of hypertension in adults with HIV initiating antiretroviral therapy in Southwestern Uganda. J Hypertens. 2015;33(10):2039.
J. J. Bigna et al., "Global burden of hypertension among people living with HIV in the era of increased life expectancy: a systematic review and meta-analysis," Journal of Hypertension, vol. 38, no. 9, 2020. [Online]. Available: https://journals.lww.com/jhypertension/Fulltext/2020/09000/Global_burden_of_hypertension_among_people_living.5.aspx.
Nguyen KA, Peer N, Mills EJ, Kengne AP. Burden, determinants, and pharmacological management of hypertension in HIV-positive patients and populations: a systematic narrative review. AIDS Rev. 2015;17(2):83–95.
Pillay S, Pillay D, Pillay RS. The burden of hypertension in KwaZulu-Natal Province, South Africa: A 6-year perspective. S Afr Med J. 2021;111(2):159–65. https://doi.org/10.7196/SAMJ.2021.v111i2.15056.
Addo J, Smeeth L, Leon DA. Hypertension in sub-saharan Africa: a systematic review. Hypertension. 2007;50(6):1012–8. https://doi.org/10.1161/HYPERTENSIONAHA.107.093336.
Egede LE, Walker RJ, Monroe P, Williams JS, Campbell JA, Dawson AZ. HIV and cardiovascular disease in sub-Saharan Africa: Demographic and Health Survey data for 4 countries. BMC Public Health. 2021;21(1):1122. https://doi.org/10.1186/s12889-021-11218-5.
Grundlingh N, Zewotir TT, Roberts DJ, Manda S. Assessment of prevalence and risk factors of diabetes and pre-diabetes in South Africa. J Health Popul Nutr. 2022;41(1):7. https://doi.org/10.1186/s41043-022-00281-2.
Jagannathan R, et al. “The OGTT is highly reproducible in Africans for the diagnosis of diabetes: Implications for treatment and protocol design,” (in eng). Diabetes Res Clin Pract. 2020;170:108523–108523. https://doi.org/10.1016/j.diabres.2020.108523.
Mesfin Belay D, et al. Diabetes mellitus among adults on highly active anti-retroviral therapy and its associated factors in Ethiopia: Systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;182:109125. https://doi.org/10.1016/j.diabres.2021.109125.
Jeremiah K, et al. “Diabetes prevalence by HbA1c and oral glucose tolerance test among HIV-infected and uninfected Tanzanian adults,” (in eng). PLoS ONE. 2020;15(4):e0230723–e0230723. https://doi.org/10.1371/journal.pone.0230723.
Sarfo FS, et al. “Prevalence and incidence of pre-diabetes and diabetes mellitus among people living with HIV in Ghana: Evidence from the EVERLAST Study,” (in eng). HIV Med. 2021;22(4):231–43. https://doi.org/10.1111/hiv.13007.
Dessie G, et al. A systematic analysis on prevalence and sub-regional distribution of undiagnosed diabetes mellitus among adults in African countries. J Diabetes Metab Disord. 2020;19(2):1931–41.
Seck SM, et al. Diabetes Burden in Urban and Rural Senegalese Populations: A Cross-Sectional Study in 2012. Int J Endocrinol. 2015;2015:163641. https://doi.org/10.1155/2015/163641.
Noubissi EC, Katte JC, Sobngwi E. Diabetes and HIV. Curr Diab Rep. 2018;18(11):125. https://doi.org/10.1007/s11892-018-1076-3.
Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304. https://doi.org/10.1136/bmjdrc-2016-000304.
Hadigan C, Kattakuzhy S. “Diabetes mellitus type 2 and abnormal glucose metabolism in the setting of human immunodeficiency virus,” (in eng). Endocrinol Metab Clin North Am. 2014;43(3):685–96. https://doi.org/10.1016/j.ecl.2014.05.003.
Ndinda C, Ndhlovu TP, Juma P, Asiki G, Kyobutungi C. The evolution of non-communicable diseases policies in post-apartheid South Africa. BMC Public Health. 2018;18:956. https://doi.org/10.1186/s12889-018-5832-8.
Gouda HN, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375–87. https://doi.org/10.1016/s2214-109x(19)30374-2.
Serenata C. Changes to the ART guidelines-an overview: guidelines. South Afr J HIV Med. 2010;11(1):28–30.
Njuguna B, Kiplagat J, Bloomfield GS, Pastakia SD, Vedanthan R, Koethe JR. Prevalence, Risk Factors, and Pathophysiology of Dysglycemia among People Living with HIV in Sub-Saharan Africa. J Diabetes Res. 2018;2018:6916497. https://doi.org/10.1155/2018/6916497.
Ojuka EO, Goyaram V. Increasing Prevalence of Type 2 Diabetes in Sub-Saharan Africa: Not Only a Case of Inadequate Physical Activity. Med Sport Sci. 2014;60:27–35. https://doi.org/10.1159/000357333.
Bigna JJ, Noubiap JJ. The rising burden of non-communicable diseases in sub-Saharan Africa. Lancet Glob Health. 2019;7(10):e1295–6. https://doi.org/10.1016/s2214-109x(19)30370-5.
Harris TG, Rabkin M, El-Sadr WM. “Achieving the fourth 90: healthy aging for people living with HIV,” (in eng). AIDS (London, England). 2018;32(12):1563–9. https://doi.org/10.1097/QAD.0000000000001870.
Muddu M, Tusubira AK, Sharma SK, Akiteng AR, Ssinabulya I, Schwartz JI. Integrated Hypertension and HIV Care Cascades in an HIV Treatment Program in Eastern Uganda: A Retrospective Cohort Study. J Acquir Immune Defic Syndr. 1999;81(5):552–61. https://doi.org/10.1097/QAI.0000000000002067.
Acknowledgements
Not applicable.
Funding
This work was supported by the AXA Research fund; and the National Institutes of Health (R01DK111848 and R01HL148271), both granted to Lara Dugas. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.M.G., J.D., C.A. and L.R.D. wrote the main manuscript. J.M.G and L.R.D. prepared figures and tables. All authors edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gizamba, J.M., Davies, J., Africa, C. et al. Prevalence of obesity, hypertension and diabetes among people living with HIV in South Africa: a systematic review and meta-analysis. BMC Infect Dis 23, 861 (2023). https://doi.org/10.1186/s12879-023-08736-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08736-5