Abstract
Introduction
Rwanda’s Hepatitis C elimination campaign has relied on mass screening campaigns. An alternative “micro-elimination” strategy focused on specific populations, such as non-communicable disease (NCD) patients, could be a more efficient approach to identifying patients and linking them to care.
Methods
This retrospective cross-sectional study used routine data collected during a targeted screening campaign among NCD patients in Kirehe, Kayonza, and Burera districts of Rwanda and patients receiving oncology services from the Butaro District Hospital. The campaign used rapid diagnostic tests to screen for Hepatitis B surface antigen (HBsAg) and Hepatitis C antibody (anti-HCV). We reported prevalences and 95% confidence intervals for HBsAg and anti-HCV, assessed for associations between patients’ clinical programs and hepatitis B and C, and reported cascade of care for the two diseases.
Results
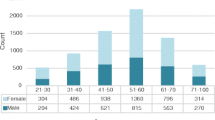
Out of 7,603 NCD patients, 3398 (45.9%) self-reported a prior hepatitis screening. Prevalence of HBsAg was 2.0% (95% CI: 1.7%-2.3%) and anti-HCV was 6.7% (95% CI: 6.2%-7.3%). The prevalence of HBsAg was significantly higher among patients < 40 years (2.4%). Increased age was significantly associated with anti-HCV (12.0% among patients ≥ 70 years). Of the 148 individuals who screened positive for HbsAg, 123 had viral load results returned, 101 had detectable viral loads (median viral load: 451 UI/mL), and 12 were linked to care. Of the 507 individuals who screened positive for anti-HCV, 468 had their viral load results returned (median viral load: 1,130,000 UI/mL), 304 had detectable viral loads, and 230 were linked to care.
Conclusion
Anti-HCV prevalence among Rwandan patients with NCD was high, likely due to their older age. NCD-HCV co-infected patients had high HCV viral loads and may be at risk of poor outcomes from hepatitis C. Hepatitis C micro-elimination campaigns among NCD patients are a feasible and acceptable strategy to enhance case detection in this high-prevalence population with elevated viral loads and may support linkage to care for hepatitis C among elderly populations.
Similar content being viewed by others
Introduction
Viral hepatitis causes over one hundred thousand deaths per year in sub-Saharan Africa [1]. Effective treatment for hepatitis B (HBV) and a functional cure for hepatitis C (HCV) are associated with decreased rates of mortality, cirrhosis, hepatic decompensation, and hepatocellular carcinoma, as well as improved production and quality of life [2]. The estimates for the global prevalence of HBV and HCV are 3.5% and 2.8%, respectively, [3] compared to 2.5% to 2.9% in Africa [4]. For HCV in particular, the recent discovery of direct acting antiretroviral (DAAs) provides a new highly efficacious treatment option that can be successfully delivered in low-resource settings [5]. For HBV, available treatments are categorized into 2 classes including interferons (standard interferon-α-2b, peginterferon-α-2a and nucleoside or nucleotide analogues (lamivudine, adefovir, entecavir, telbivudine, tenofovir disoproxil fumarate, and tenofovir alafenamide), which are effective in suppressing HBV replication but do not eradicate the virus [6, 7]. Despite the availability of treatments for both HBV and HCV, most people remain unaware of their hepatitis status until symptoms appear, resulting in liver damage and poor health outcomes [8].
In light of the burden of viral hepatitis and the availability of effective treatment options, Rwanda launched a 5-year strategic plan for HCV elimination by 2024 [9]. This plan, which involves both case identification and treatment of identified cases, relies heavily on providing widespread testing for both HBV and HCV, which has largely been conducted through mass screening campaigns that target the general population [10, 11]. However, HCV elimination strategies that use mass screening approaches among the general population is daunting, complex and highly priced with cost estimates running in the billions of euros of in high-income countries [12] and 45 million dollars in Rwanda along [13], and billions of euros One alternative approach is a “micro-elimination” strategy, which breaks down national elimination goals into smaller, achievable goals within specific segments of the population so that targets can be achieved in a shorter period of time with fewer financial resources [14, 15].
One potential target population for hepatitis micro-elimination is non-communicable disease (NCD) patients. These patients may be at elevated risk for HCV due to their older average age, which is a risk factor that is associated with HCV and with many common NCDs [16, 17]. For example, a recent study in Butaro found that the prevalence of HCV infection was found to be 9.4% among individuals aged 45 and above [18]. Additionally, some studies suggest that chronic HCV infection can cause extrahepatic conditions, such as type 2 diabetes, Circulatory diseases, and Chronic kidney diseases (CKD) [19,20,21]. Furthermore, because NCD patients are already in regular contact with the healthcare system, they may be more easily mobilized for screening and linkage to treatment compared to other high-risk populations.
Although Rwanda has conducted limited targeted screening among people living with human immunodeficiency virus (HIV), pregnant women [10, 22], prisoners, sex workers, injection drug users, and men who have sex with men (MSM) [23], NCD patients are not specifically targeted for hepatitis screening by the national Rwandan hepatitis program. In this context, our team implemented a targeted HBV and HCV screening among patients with NCDs in rural setting. This paper reports on the sero-prevalence and associated risk factors for HBsAg and anti-HCV among NCD patients in three rural Rwandan districts as well as the cascade of care and viral load results observed among patients who screened positive.
Methods
Study design
This study is a retrospective cross-sectional study used routine data collected during the screening of HBV and HCV among NCD patients in three rural districts of Rwanda.
Setting
The screening program was led by Partners In Health/Inshuti Mu Buzima (PIH/IMB), an international non-government organization that supports health care implementation in three rural Rwandan districts (Kirehe, Kayonza and Burera) in partnership with the Rwandan Ministry of Health. PIH/IMB supports Kirehe, Rwinkwavu, and Butaro district hospitals together with their 44 affiliated health centers. The screening campaign were conducted from November 2020 to March 2021.
Participants
All NCD patients aged 15 years and above receiving care at health facilities within the Kirehe, Butaro and Rwinkwavu(in Kayonza) District Hospitals’catchment areas were invited to participate in a voluntary mass screening campaign for HBV and HCV. In accordance with national guidelines for hepatitis screening and treatment [24], patients were excluded from screening if they were under 15 years of age or if they had already been initiated on treatment for HBV or HCV. However, some patients who had already been initiated on treatment were provided with viral load testing if the timing of the screening aligned with clinical guidelines for viral load monitoring [24]. Oncology patients who received out-patient or in-patient oncology services from the Butaro District Hospital Cancer Center of Excellence during the screening campaign were also eligible to participate. However, because the Cancer Center serves patients from around the country, and some of them often travel long distances to the Cancer Center, oncology patients were not invited to attend screening unless a screening day coincided with an existing appointment. Non-NCD patients at the health center, such as patients receiving care for mental health, were provided with screening on request in the interest of furthering the national elimination targets and providing equitable access to healthcare, but were not explicitly invited to participate.
Screening campaign
Community awareness for the campaign was initially done through meetings with local leaders, the director general from each district hospital, the heads of health centers, and local community health workers. In collaboration with the NCD program of PIH/IMB, a list of NCD patients attending each health facility was generated and patients were informed of the date when the campaign would come to their nearest health facility via telephone or community health worker.
Prior to testing, we conducted a group education, information, and communication session regarding viral hepatitis and obtained verbal consent for HBV and HCV screening. During screening, trained nurses and laboratory technicians collected capillary blood samples to test for anti-HCV and HBsAg using SD Bioline rapid diagnostic tests (RDTs). These tests are among rapid diagnosis tests that are pre-approved by World Health Organization(WHO) and are manufactured by Abbott Diagnostics Korea Inc, Giheung-gu, Korea [25, 26], and have a sensitivity and specificity of > 99% to detect anti-HCV and sensitivity of 96.7% and specificity of 98.9% to detect HBsAg [27]. For patients who screened positive, 4-5 ml of blood for HBV Deoxyribonucleic Acid (DNA) and/or HCV Ribonucleic Acid (RNA) was drawn from each participant through venipuncture using a vacutainer and an ethylene-diamine-tetra-acetic acid (EDTA) anticoagulant tube for viral load testing.
The collected blood samples from health facilities in Kirehe and Kayonza catchment areas were transported to Kirehe District Hospital every day, while those from Burera District were transported to Ruhengeri district hospital for viral load testing. These two hospitals are among the closest district hospitals in the country that have the necessary equipment for hepatitis viral load testing. During transportation, samples were placed in the tube rack, and put into cooler box for triple packing as recommended [28, 29]. Blood samples were centrifuged (Universal 320 R) at 3000 rpm to separate plasma from whole blood and plasma was used for viral load testing. If the plasma was not analyzed on the same day, it was kept at -200C until analysis is done. Viral load testing was performed using COBAS 480 HCV and HBV Test, V.2.0: Quantitative (Roche) with a lower limit of quantification of 15 IU/mL and 10 IU/Ml for HCV and HBV, respectively.
Variables & data sources
This analysis used secondary data that were collected during patient registration, screening, and viral load testing. During the screening campaign, data was recorded into Research Electronic Data Capture (REDCap) database for operational purposes and used to facilitate patient follow-up.
Social demographic information was collected during patient registration process using a digitalized form programmed into REDCap. Before analysis, duplicate patient entries and patient who had previously screened positive and were already on treatment for hepatitis were removed from analysis. Names, identifying numbers, and address were removed from the dataset before analysis to maintain patient health records confidentiality.
Study size
The targeted screening campaign was expected to cover 9,920 NCD patients as well as any oncology patients receiving out-patient or in-patient oncology services from the Butaro District Hospital Cancer Center of Excellence during the screening campaign. Of the 8,125 individuals who attended the targeted screening campaign, 7,622 were NCD patients, reflecting a screening coverage of 76.7% among NCD patients, 160 were oncology patients, and 344 were other visitors at the health center who requested a screening.
Statistical methods and data analysis
We described demographic characteristics of patients using percentages and frequencies. We calculated prevalence and 95% confidence intervals for HBsAg and anti-HCV in the overall population of patients who participated in the screening campaign. We assessed bivariate associations between clinical NCD program and prevalence of HBsAg and anti-HCV using Fisher’s exact or Chi-squared tests. Because age and sex are known to be strong risk factors for viral hepatitis in Rwanda, we used multivariate logistic regressions to determine the relationship between the demographic characteristics and HBsAg and anti-HCV after adjusting for age and sex.
To understand whether patients with NCD were at higher risk of hepatitis compared to the general population, we also compared prevalence among NCD patients with the recent results from the nationally-representative Rwanda Population-Based HIV Impact Assessment(RPHIA) [30], which published prevalence of HBsAg and anti-HCV among Rwandans aged 15–64. We calculated the crude prevalence of HBsAg and anti-HCV among the subset of NCD patients who were 15–65 years of age. We also directly standardized to the age distribution of RPHIA survey respondents using 5-year age categories. This directly standardized prevalence can be interpreted as the expected prevalence that we would have observed if the NCD patients had the same distribution of ages as the RPHIA participants. The age distribution of our screening participants as well as the reference population is given Table 5 in Appendix 1.
Among patients who screened positive, we also describe the cascade of care by reporting the counts and frequencies of patients who had their viral load results returned, had detectable viral loads, and were linked to care. For patients with detectable viral loads (102 for HBV and 304 for HCV), we reported the median and interquartile ranges (IQR) for viral loads.
Results
Of the 8,125 individuals who participated in screening, we excluded 198 patients with a prior diagnosis of hepatitis C and 324 additional patients who did not belong to the NCD or oncology program leaving a total of 7,603 screening participants to be included in analysis. Half of all participants were from Burera district (n = 3813, 50.2%), a third from Kirehe district (n = 2326, 30.6%), and the remaining from Kayonza District (n = 1460, 19.2%), (Table 1). The majority of NCD patients were female (n = 5,978, 78.7%), over 60 years (n = 4488, 59.8%), were married or cohabitating (n = 4752, 62.7%), and had received less than a primary education (n = 6032, 81.4%). The plurality was in Ubudehe 3 or 4 (n = 3,077, 41.0%) and the overwhelming majority of participants were using community-based health insurance (CBHI) commonly called Mutuelle (n = 7,358; 97.9%). Patients were most likely to be enrolled in the NCD program for hypertension (n = 6032, 81.4%) almost half of participants self-reported being previously screened for hepatitis (n = 3398, 45.9%) (Table1).
Prevalence of HBsAg was 2.0% (95% CI: 1.7%-2.3%) and anti-HCV was 6.7% (95% CI: 6.2%-7.3%). HBsAg was not associated with any clinical program in either the crude of adjusted analyses (Table 2). Anti-HCV prevalence was higher in the hypertensive patients compare to other NCD programs (6.6% vs 5.7%), but this difference was not statistically significant after adjusting for age and sex (OR = 1.1, 95% CI: 0.9, 1.5, p = 0.358 (Table 2).
When we restricted our sample to ages 15–64 and directly standardized our age distribution to the ages of respondents to the Rwandan Population-based HIV Impact Assessment 2018–2019, we observed that, after adjusting for age, NCD patients presented similar risks of anti-HBsAg and anti-HCV compared to the general population (Table 3).
Of the 148 individuals who screened positive for HbsAg, 123 (83%) had their viral load results returned and recorded, 101 (82%) of these had detectable viral loads, and 12 (12%) successfully linked to care (Fig. 1). Median detectable viral load among individuals with detectable HBV was 451 UI/mL (IQR: 166–1860) (Table 4). Of the 507 individuals who screened positive for anti-HCV, 468 (92%) had their HCV viral load results returned and recorded, 304 of these (65%) had detectable viral loads and were eligible for treatment, and 230 (76%) were linked to care (Fig. 2). Median detectable viral load among individuals with detectable hepatitis C viral load was 1,130,000 UI/mL (IQR: 323,000–2,700,000) (Table 4).
Discussion
In this study, we examined HBsAg and anti-HCV sero-prevalences. We assessed risk factors associated with HBsAg and Anti-HCV among NCD and oncology patients participating in a targeted screening campaign in three rural Rwandan districts. Overall, HBsAg prevalence among NCD patients who participated in our screening campaign (2.0%) was comparable to previous reports from RPHIA 2.0% [30]. Similar to the findings of other studies conducted in Rwanda [18, 31], our research revealed a potential higher risk of HBV infection among males in our study population when compared to females.
The overall prevalence of anti-HCV among NCD patients was 6.7%, which is much higher than the 1.2% prevalence of anti-HCV reported in RPHIA. These finding likely stem from the strong association between age and hepatitis C, which has been repeatedly found to be a major risk factor for anti-HCV [16, 17, 32]. Thirty percent of patients who participated in this targeted NCD screening campaign were over the age of 70, making them meaningfully older than both the general population in Rwanda and the RPHIA respondents.
After standardizing to account for the advanced age of the NCD patients, we found that the risk of HBsAg among NCD patients compared to the general population was slightly lower overall (1.8% vs. 2.0%) and among men (2.4% vs. 2.8%) but slightly higher among women (1.7% vs. 1.3%). NCD patients were at slightly lower risk of anti-HCV overall (1.0 vs. 1.2%), among men (0.9% vs. 1.3%) and among women (1.0% vs. 1.1%). Similarly, despite the fact that A. Lecube et al., [19], and M. Basaranoglu et al., [33] have previously reported that HCV infection is associated with diabetes mellitus, we did not find any evidence that any NCD diagnosis was associated with hepatitis after adjusting for age and sex. Collectively, these findings suggest that NCD patients face an elevated risk of HCV compared to other Rwandans due to their advanced age rather than due to other NCD-related factors or individual behaviors.
While the risk of HCV among NCD patients in Rwanda is similar to among other elderly Rwandan populations [18, 34, 35], the high overall prevalence of HCV means that, they are still strong candidates for a targeted screening campaign. Furthermore, there is some evidence to suggest that the NCD patients in our campaign may be at elevated risk of poor health outcomes from HCV. Although the proportion of anti-HCV positive patients with a detectable viral load was lower (65%) than what has been reported elsewhere in Rwanda (78%) [36], the median hepatitis C viral load among NCD patients observed in this study was high (1,130,000UI/ML). Moucar R et al., [37] conducted a study that demonstrated that Insulin Resistance was associated with a higher serum HCV RNA level. This suggests that there may be biological mechanisms that make NCD-HCV co-infected patients more vulnerable to high HCV viral loads and more susceptible to subsequent poor health outcomes compared to the patients with HCV alone.
Our study also shows that almost half of NCD patients self-reported prior hepatitis screening. However, a history of previous screening was not associated with a reduced risk of disease. This pattern of repeated screening and prevalent hepatitis among repeat screeners suggests a suboptimal linkage to hepatitis treatment. Furthermore, during our campaign, many NCD patients reported having been previously diagnosed with hepatitis infection, but they often did not know which type of hepatitis infection they had. This lack of knowledge may prevent patients from accessing appropriate care, as has been discussed previously [38]. Investing in high-quality electronic data collection during screening campaigns, using mobile hepatitis treatment clinics to provide decentralized care, a three strategies that we have used to successfully mitigate those problems [36, 39]. However, even within our targeted screening campaign, there were substantial gaps in linkage to care, particularly among NCD patients who screened positive for HBsAg. Our previous research has identified that incomplete collection of patient contact information, delays in turn-around times for viral load testing, and costs associated with accessing pre-treatment-initiation lab tests are important barriers to linking patients to timely treatment for viral hepatitis [36, 40]. Embedding hepatitis screening campaigns within existing chronic care programs, such as the NCD program, may mitigate some of these challenges because the patients have existing relationships and repeated points of contact with the health care system. However, investing in more efficient laboratory processes, and healthcare provider training will be necessary to ensure that access to hepatitis diagnosis, and treatment are truly accessible.
Our work does have some limitations. First, our analysis relies on routine data collected during the screening campaign, and there was no comprehensive questionnaire to collect information on all known risk factors for hepatitis. This routine data suffers from some inaccuracies or missingness, particularly around the return of viral load results and linkage to care. Second, this study was conducted within the catchment areas of three rural Inshuti Mu Buzima (IMB)-supported Rwandan district hospitals and as such may have limited generalizability. However, given that most district hospitals in Rwanda and in the region are located in rural settings with similar population characteristics, these results can likely be extrapolated to other similar settings in Rwanda or elsewhere.
Conclusion
Our findings do suggest that NCD patients are good target populations for hepatitis C micro-eliminiation campagins in Rwanda. First, most NCD patients are at elevated risk of hepatitis C due to their advanced age. Second, they may suffer more severe outcomes from hepatitis due to higher viral loads among NCD-HCV co-infected patients. Third, in settings where linkage to care among the general population remains challenging, targeting NCD patients who already have established connections to the healthcare system appears to be an acceptable opportunity for mobilizing the community and may enhance linkage to care for elderly patients. While NCD patients are not at higher risk of HBV, including HBV screening and linkage to treatment as part of HCV elimination campaigns is both feasible and acceptable. Because increased age is associated with HCV in many settings, the micro-elimination of HCV among NCD pateints is a strategy that could be considered in a wide variety of settings.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author or the Inshuti Mu Buzima Research committee (imbrc@pih.org) on reasonable request.
Abbreviations
- CBHI:
-
Community Health Insurance
- CI:
-
Confidence Interval
- DAAs:
-
Direct Acting Antivirals
- HCV:
-
Hepatitis C Virus
- IHDPC:
-
Institute of HIV Disease Prevention and Control
- IMBRC:
-
Inshuti Mu Buzima Research Committee
- IQR:
-
Interquartile range
- PIH/IMB:
-
Partners In Health/Inshuti Mu Buzima
- LMIC:
-
Low and Middle Income Countries
- MoH:
-
Ministry of Health
- SVR12:
-
Sustained Virologic Response after 12 weeks
- RNEC:
-
Rwanda National Ethics Committee
- RDT:
-
Rapid Diagnostic Test
- RBC:
-
Rwanda Biomedical Centre; REDCap: Research Electronic Data Capture
- RNA:
-
Ribonucleic Acid
- WHO:
-
World Health Organization
- HBsAg:
-
Antibody to Hepatitis B surface antigen
- HBV:
-
Hepatitis B Virus
- HCVab:
-
Hepatitis C Virus antibody
- DNA:
-
Deoxyribonucleic acid
- HIV:
-
Human Immunodeficiency Virus
- NCDs:
-
Non-Communicable Diseases
- RPHIA:
-
Rwanda Population-Based HIV Impact Assessment
- MSM:
-
Men who have Sex with Men
- REDCap:
-
Research Electronic Data Capture
References
Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–8.
Ghany MG, Morgan TR. Hepatitis C guidance 2019 update: American association for the study of liver diseases-infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686–721.
Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016;22(10):833–8. https://doi.org/10.1016/j.cmi.2016.07.035.
Petruzziellorn A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–40.
Nsanzimana S, Penkunas MJ, Liu CY, Sebuhoro D, Ngwije A, Remera E, et al. Effectiveness of direct-acting antivirals for the treatment of chronic hepatitis C in Rwanda: a retrospective study. Clin Infect Dis. 2020;110(8):2019.
Vittal A, Mhsc MGG. WHO guidelines for p re v e n t i o n, c a re a n d tre a t m e n t o f i n d i v i d u a l s i n f e c t e d with HBV. Clin Liver Dis. 2019;23(3):417–32. https://doi.org/10.1016/j.cld.2019.04.008.
Suk-Fong LA. Hepatitis B treatment: what we know now and what remains to be researched. Hepatol Commun. 2019;3(1):8–19.
Mauss S, Pol S, Buti M, Duffell E, Gore C, Lazarus JV, et al. Late presentation of chronic viral hepatitis for medical care: a consensus definition. BMC Med. 2017;15(1):1–5.
Umutesi G, Shumbusho F, Kateera F, Serumondo J, Kabahizi J, Musabeyezu E, Ngwije A, Gupta N, Nsanzimana S. Rwanda launches a 5-year national hepatitis C elimination plan: A landmark in sub-Saharan Africa. J Hepatol. 2019;70(6):1043–5. https://doi.org/10.1016/j.jhep.2019.03.011.
Umutesi J, Simmons B, Makuza JD, Dushimiyimana D, Mbituyumuremyi A, Uwimana JM, et al. Prevalence of hepatitis B and C infection in persons living with HIV enrolled in care in Rwanda. BMC Infect Dis. 2017;17(1):1–7.
Umutesi J, Liu CY, Penkunas MJ, Makuza JD, Ntihabose CK, Umuraza S, et al. Screening a nation for hepatitis C virus elimination: a cross-sectional study on prevalence of hepatitis C and associated risk factors in the Rwandan general population. BMJ Open. 2019;9(7):1–8.
Lazarus JV, Wiktor S, Colombo M, Thursz M. Micro-elimination – A path to global elimination of hepatitis C. J Hepatol. 2017;67(4):665–6. https://doi.org/10.1016/j.jhep.2017.06.033.
Makuza JD, Tuyishime A, Janjua N, Gupta N. HBV elimination in sub-Saharan Africa: Rwanda’s approach to health system integration. Lancet Gastroenterol Hepatol. 2022;7(6):511–2. https://doi.org/10.1016/S2468-1253(22)00134-0.
Lazarus JV, Wiktor S, Colombo M, Thursz M. Micro-elimination – A path to global elimination of hepatitis C. J Hepatol. 2017;67:665–6 Elsevier B.V..
Lazarus JV, Safreed-Harmon K, Thursz MR, Dillon JF, El-Sayed MH, Elsharkawy AM, et al. The micro-elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis. 2018;38(3):181–92.
Makuza JD, Rwema JOT, Ntihabose CK, Dushimiyimana D, Umutesi J, Nisingizwe MP, et al. Prevalence of hepatitis B surface antigen (HBsAg) positivity and its associated factors in Rwanda. BMC Infect Dis. 2019;19(1):1–10.
Nahimana M, Nyandwi A, Muhimpundu MA, Olu O, Condo JU, Rusanganwa A, et al. A population-based national estimate of the prevalence and risk factors associated with hypertension in Rwanda: implications for prevention and control. 2018. p. 1–11.
Iradukunda PG, Habyarimana T, Niyonzima FN, Uwitonze AY, Mpunga T. Risk factors associated with hepatitis B and C in rural population of Burera district, Rwanda. Pan Afr Med J. 2020;35:1–10.
Lecube A, Hernández C, Genescà J, Simó R. Glucose abnormalities in patients with hepatitis C virus infection: epidemiology and pathogenesis. Diabetes Care. 2006;29(5):1140–9.
Chen WJ, Chen C; Group REVEALS. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases : a community-based long-term prospective study. J Infect Dis. 2012;206:469–77.
Rossi C, Raboud J, Walmsley S, Cooper C, Antoniou T, Burchell AN, et al. Hepatitis C co-infection is associated with an increased risk of incident chronic kidney disease in HIV-infected patients initiating combination antiretroviral therapy. BMC Infect Dis. 2017;17(1):1–10.
Mutagoma M, Balisanga H, Sebuhoro D, Mbituyumuremyi A, Remera E, Malamba SS, et al. Hepatitis C virus and HIV co-infection among pregnant women in Rwanda. 2017. p. 4–9.
Umutesi J, Klett- C, Nsanzimana S, Krause G. sectional study of chronic hepatitis B virus infection in Rwandan risk groups: unexpected findings on prevalence and its determinants. 2021.
Marcuccilli F, Chevaliez S, Muller T, Colagrossi L, Abbondanza G, Beyser K, et al. Multicenter evaluation of the cepheid xpert® hbv viral load test. Diagnostics. 2021;11(2):1–7.
Shenge JA, Osiowy C. Rapid diagnostics for hepatitis B and C viruses in low- and middle-income countries. Front Virol. 2021;1(October):1–12.
Jargalsaikhan G, Eichner M, Boldbaatar D, Bat- P. Sensitivity and specificity of commercially available rapid diagnostic tests for viral hepatitis B and C screening in serum samples. 2020;1–9. https://doi.org/10.1371/journal.pone.0235036
Health MOF. Ministry of health national reference laboratory biological sample transportation within the national laboratory. 2012.
Shrestha LB, Pokharel K. Standard Operating Procedure for Specimen Collection, Packaging and Transport for Diagnosis of SARS-COV-2. JNMA J Nepal Med Assoc. 2020;58(228):627–9. https://doi.org/10.31729/jnma.5260.
Rwanda Biomedical Center (RBC). Rwanda Population-Based HIV Impact Assessment. (RPHIA) 2018-2019: Final Report. Kigali: RBC; 2020.
Nisingizwe MP, Makuza JD, Janjua NZ, Bansback N, Hedt-Gauthier B, Serumondo J, et al. The cascade of care for hepatitis C treatment in Rwanda: a retrospective cohort study of the 2017–2019 mass screening and treatment campaign. Viruses. 2023;15(3):661.
Carvalho-Gomes Â, Cubells A, Pallarés C, Hontangas V, Conde I, Di MT, et al. A population-based screening for hepatitis C antibodies and active infection using a point-of-care test in a low prevalence area. PLoS ONE. 2020;15(2):1–14.
Basaranoglu M, Basaranoglu G. Pathophysiology of insulin resistance and steatosis in patients with chronic viral hepatitis. World J Gastroenterol. 2011;17(36):4055–62.
Makuza JD, Liu CY, Ntihabose CK, Dushimiyimana D, Umuraza S, Nisingizwe MP, et al. Risk factors for viral hepatitis C infection in Rwanda: results from a nationwide screening program. 2019. p. 1–10.
Pierre G, Iradukunda PG, Tumusiime M, Harelimana JDD, Rutayisire G, Nyakatswau ST, et al. Medical laboratory professional’s week in Rwanda: a field report from the Simbi and Maraba communities. Int J Community Med Public Heal. 2021;8(5):2111.
Id IK, Barnhart DA, Ndahimana A, Noor K, Mumporeze J, De J, et al. Prevalence and associated risk factors for hepatitis B and C viruses among refugee populations living in Mahama, Rwanda: a cross-sectional study. 2021. p. 1–14.
Hcv S, Level RNA, Fibrosis L, Hélène M, Chanoine N, Paradis V, et al. Insulin resistance in chronic hepatitis C: association with genotypes. 2008. p. 416–23.
Barnhart DA, Kamali I, Nyirahabihirwe F, Mugabo C, De J, Gakuru P, et al. Knowledge among patients with Hepatitis C initiating on direct-acting antiviral treatment in rural Rwanda: a prospective cohort study. Glob Health Action. 2021;14(1):1953250. https://doi.org/10.1080/16549716.2021.1953250.
Nyirahabihirwe F, Kamali I, Barnhart DA, Gakuru J de la P, Musafiri T, Rwamuhinda DD, et al. Implementation of Refugees’ Inclusion in National Viral Hepatitis B and Hepatitis C Screening Campaign in Mahama Refugee Camp, Rwanda. Glob Heal Sci Pract. 2022;10(2):e2100349. http://www.ghspjournal.org/content/10/2/e2100349.abstract
Kamali I, Barnhart DA, Nyirahabihirwe F, de la Paix Gakuru J, Uwase M, Nizeyumuremyi E, Walker S, Mazimpaka C, de Dieu Gatete J, Makuza JD, Serumondo J, Kateera F, d'Amour Ndahimana J. Initiation of hepatitis C treatment in two rural Rwandan districts: a mobile clinic approach. BMC Infect Dis. 2021;21(1):220. https://doi.org/10.1186/s12879-021-05920-3.
World Medical Association. WMA Declaration of Helsinki: ethical principles for medical research involving human subjects. 1974;353(1):1418–9. Available from: http://www.wma.net/en/30publications/10policies/b3/index.html
Acknowledgements
We would like to thank the support of Partners In Health/Inshuti Mu Buzima for this work. The study was developed under the fulfilment of the requirements for the Master’s degree in Public Health at Mount Kenya University Rwanda. Also, Iwould like to express my special appreciation and thanks to my supervisors and mentors; Theoneste Ntakirutimana, Casmille Kayihura, Kamali Innocent and Dale A. Barnhart for providingdirect mentorship to this paper as part of capacity building in Research.
Funding
DAB is supported by the Harvard Medical School Global Health Equity Research Fellowship, funded by Jonathan M. Goldstein and Kaia Miller Goldstein. The data collection, analysis, interpretation and writing the manuscript were conducted independently by authors.
Author information
Authors and Affiliations
Contributions
TM is the principle author of this manuscript in all steps from the development of the study protocol. DAB, JDA, and TN conceptualized the study. TM led the literature search and manuscript writing. DAB, and IK, PH led data cleaning and analysis and provide mentorship for paper development. TM, IK, JDA, DAB,TN, CK, JG, FN, PK,PH, RS, MJD, NK, JS, and EN supported in the integration of data collection into clinical activities and assisted in the interpretation of the findings. DAB, JDA, IK, TN oversaw study design, and provide mentorship to PK, PH and TM who contributed significantly in data analysis and developed the figure and tables. Al authors reviewed and provided critiques of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study approval was obtained from Rwanda National Ethics Committee (RNEC) 015/RNEC/2020), Inshuti Mu Buzima Research Committee (IMBRC), and Mount Kenya University’s Ethic Committee. Because this study used retrospective data, which was collected as part of routine clinical practice; therefore, informed consent was not required by the Rwanda National Ethics Committee. The appropriate guideline [41], under the subject of "Ethics approval and consent to participate," was followed in full.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Table 5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Musafiri, T., Kamali, I., Kayihura, C. et al. Prevalence of hepatitis B and C infection and linkage to care among patients with Non-Communicable Diseases in three rural Rwandan districts: a retrospective cross-sectional study. BMC Infect Dis 24, 247 (2024). https://doi.org/10.1186/s12879-023-08678-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08678-y