Abstract
Background
Scedosporium is a lesser-known non-Aspergillus genus of mold that can present in unsuspecting ways. If overlooked, it may disseminate and cause high mortality in high-risk allogeneic stem cell transplant recipients.
Case presentation
This case report describes a 65-year-old patient with Acute Myeloid Leukemia who underwent an allogeneic hematopoietic stem cell transplant after a period of prolonged neutropenia with fluconazole prophylaxis. She suffered severe debility with altered mentation from a S. apiospermum infection which likely disseminated from a toe wound to the lung and central nervous system. She was successfully treated with liposomal amphotericin B and voriconazole, but faced a prolonged recovery from physical and neurologic sequela.
Conclusions
The case highlights the importance of adequate anti-mold prophylaxis in high-risk patients, and the value of a thorough physical examination in this patient population, with particular attention to skin and soft tissue findings.
Similar content being viewed by others
Background
Allogeneic hematopoietic stem cell transplant (alloHCT) offers a potential cure for poor-risk acute myeloid leukemia (AML) and other hematologic malignancies [1]. The need for immunosuppressive therapy, in conjunction with prolonged neutropenia during and often before transplantation, places the recipient at risk for invasive mold infections. Antifungal prophylaxis sometimes does not include anti-mold prophylaxis. As a result, molds like Aspergillus, implicated in up to 80% of invasive fungal disease (IFD), can complicate alloHCT [2]. Scedosporium is a lesser-known ubiquitous mold that has increasingly been associated with disseminated infections and poor survival [3, 4].
We describe a case of disseminated Scedosporium infection in a patient with AML who developed pulmonary and central nervous system (CNS) fungal infection from an unexpected source (right hallux) after alloHCT and was treated successfully. The aim of this case presentation is to reinforce the necessity of adequate anti-mold prophylaxis in patients with prolonged neutropenia prior to transplantation, and to highlight the importance of a thorough “head to toe” physical examination.
Case
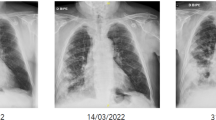
The patient is a 65-year-old female who was diagnosed with unfavorable risk AML [with TP53 mutation and del(5q)] in April 2022. She was refractory to standard induction therapy but responded to a combination of a hypomethylating agent and venetoclax (3 cycles). She remained neutropenic throughout and after treatment, and received prophylaxis with fluconazole 400 mg daily for 3 months until her transplant, due to concern for elevated liver enzymes previously with posaconazole. She was neutropenic upon admission for alloHCT [white blood cells (WBC) 0.81 × 109/L and absolute neutrophil count (ANC) 0.62 × 109/L]. Her pre-transplant chest computerized tomography (CT) revealed irregular nodular opacities, therefore posaconazole 300 mg daily was re-challenged on admission (day -6). On day + 5, she was switched to micafungin 150 mg daily with concern for transaminitis. On day + 6, she developed hypoxemia, and had a worsening RUL nodular opacity on chest CT (Fig. 1A, B). Bronchioalveolar lavage (BAL) was performed with negative BAL galactomannan and cultures. Serum β-D-Glucan (Fungitell™ assay) was positive (180 pg/mL, reference range < 60 pg/mL). Her hypoxia resolved with diuresis, and posaconazole was resumed day + 8. On day + 15, neutrophil engraftment was noted and was associated with hypoxia, fevers, and diarrhea. Methylprednisolone (1 mg/kg daily) started on day + 16 for engraftment syndrome led to improvement.
CT chest. Non-contrast computerized tomography (CT) showing right upper lobe nodular opacity 20 days before transplant, measuring 0.4 cm (A), and on day + 6 after transplant, measuring 1.6 × 1.8 cm (B). The pre-transplant opacities were of indeterminate significance, and attributed to possible resolving infection
At the same time, she was noted to have right toe swelling and erythema, initially thought to be traumatic and improved without intervention (Fig. 2A). Onychomycosis was presumed due to the appearance of the nailbed, and topical ketoconazole 2% ointment was applied for the duration of her treatment course. Her performance status declined significantly, and she became more lethargic and less responsive to verbal or non-verbal stimuli. Viral studies including serum human herpesvirus 6 (HHV6), adenovirus, Epstein-Barr virus (EBV), cytomegalovirus (CMV), and blood cultures, were unrevealing. CT of the brain was negative for bleeding or any new abnormality to explain the clinical picture. Psychiatry and neurology evaluations suggested hypoactive delirium and medications were adjusted to account for possible psychogenic side effects. On day + 26, with no improvement in her mental status, brain magnetic resonance imaging (MRI) showed a rim-enhancing lesion in the periventricular right frontoparietal region, concerning for abscess (Fig. 3A). Cerebral spinal fluid (CSF) PCR studies for CMV, Herpes simplex, HHV6, adenovirus, EBV, Cryptococcus, and fungal and bacterial cultures were negative, though protein and glucose were elevated. CT chest was suggestive of persistent but improved nodular opacities. Dual antifungal therapy comprising liposomal amphotericin B 3 mg/kg daily and voriconazole 350 mg BID was started. Repeat imaging a week later revealed an increase in size of the abscess with interval new lesions (Fig. 3B). The liposomal amphotericin B dose was increased to 5 mg/kg daily while voriconazole level was therapeutic at 2.8 µg/mL. Her level of alertness waxed and waned; however, she remained bedbound and required enteral nutrition. With no clear improvement, CSF analysis was repeated and remained negative for all pathologic microbiologic agents (repeated as above). Brain biopsy was deferred due to high risk for complications. Her right toe exam was concerning for worsening paronychia. Podiatry noted a positive probe-to-bone test, a finding with greater than 90% positive predictive value for osteomyelitis in high-risk patients, but lower in immunocompetent patients [5]. This finding was further supported by decreased T1 marrow signal intensity on MRI (Fig. 2B), a test with a lower positive predictive value approaching 80% [6]. The probed tissue was cultured, and initially grew coagulase-negative Staphylococcus and diptheroids. She received doxycycline 100 mg BID for 4 weeks.
After 5 weeks of aggressive antifungal therapy (day + 58), MRI imaging finally showed significant improvement in the brain abscess. On day + 60, fungal cultures from the right hallux grew Scedosporium apiospermum, 7 days after specimen collection. Culture media included Sabouraud Dextrose with brain–heart infusion agar, inhibitory mold agar, and brain–heart infusion agar with sheep blood, chloramphenicol, and gentamycin. Laboratory identification was performed at the University of Texas Health Science Center fungus testing laboratory, based on sequencing of internal transcribed spacer, beta-tubulin, and calmodulin loci. Antifungal susceptibilities (using CLSI M38-A2 standard) showed voriconazole minimum inhibitory concentrations (MIC) = 1 µg/ml, posaconazole MIC = 1 µg/mL, amphotericin B MIC = 1 µg/ml and isavuconazole MIC = 8 µg/ml. It should be noted that despite the low MIC, liposomal amphotericin B clinically performs poorly against Scedosporium spp. regardless of the MIC range [7]. Liposomal amphotericin B was discontinued and voriconazole continued as monotherapy. At day + 76, her poor performance status remained unchanged despite improvement in her imaging and otherwise excellent organ and graft function, and she was transferred to a rehabilitation facility. A repeat MRI brain on day + 83, after 8 weeks of treatment, showed significant decrease in the size of the lesion (Fig. 3C). Her mental status and cognitive functions eventually returned to baseline during her rehabilitation course. However, she developed failure to thrive due to other non-infectious HCT complications, and opted to transition to comfort care. She ultimately passed away 6 months after her HCT. See Fig. 4 for a timeline of the patient’s clinical course and interventions.
Timeline of clinical course. CT; computerized tomography; MRI, magnetic resonance imaging; BAL, bronchioalveolar lavage; CSF, cerebrospinal fluid aNegative for pneumocystis, galactomannan, listeria, bacterial, AFB, and fungal cultures, and respiratory viral pathogens bHuman Herpesvirus (HHV)-6, cytomegalovirus, adenovirus, Epstein-Barr virus (EBV) undetectable CNegative for cytomegalovirus, herpes simplex 1/2, HHV-6, adenovirus, EBV, cryptococcus, fungal, AFB, and bacterial cultures. WBC 1/uL, 15 total cells (93% lymphocytes), glucose elevated in the first sample, 82 mg/dL; Protein elevated in both samples, 75.4 mg/dL and 47.4 mg/dL, respectively
Discussion
Scedosporium apiospermum is one of the more common species of non-Aspergillus molds detected in the alloHCT population and is a significant cause of mortality [4]. Our patient possessed several risk factors that predisposed her to this infection. She received a transplant from a matched unrelated donor; did not receive anti-mold prophylaxis despite prolonged neutropenia prior to transplantation, and received high-dose corticosteroid therapy [8]. Moreover, we later learned that her home environment was unsanitary and a possible source for skin inoculation. Disseminated Scedosporium spp. can originate from a wide range of local sites [9]; in this case, it possibly began as osteomyelitis of her right hallux despite the classic appearance of paronychia. Several cases of disseminated disease originating from skin/soft tissue infections and osteomyelitis are reported in the literature [10, 11]. Another possibility is inhalation of S. apiospermum with secondary dissemination to the brain and soft tissue. Scedosporium spp. have low reactivity to the β-D-Glucan assay, but as in this case, a positive test can still occur and may suggest the diagnosis particularly in cases where an invasive fungal infection is suspected but undiagnosed [12]. In this case, surgical sampling of tissue was considered but deferred due to the patient’s high risk of surgical complications. Fungal blood cultures were also negative. Therefore, in the absence of tissue sampling, we cannot prove the diagnosis of a disseminated infection versus a contaminant. However, given the host risk factors, clinical manifestations of lung, brain, and skin involvement, isolation of Scedosporium on culture, and serum β-D-Glucan positivity, a diagnosis of disseminated Scedosporium is favored, as further supported by continued clinical improvement on voriconazole monotherapy.
Newer triazoles, particularly voriconazole, have been shown to have superior in vitro activity against S. apiospermum compared to older triazoles, amphotericin B, and echinocandins [9], and have been associated with better survival in retrospective studies [3]. Isavuconazole does not appear to be as effective as voriconazole. The management of this patient was in line with current guidelines, which are strongly in favor of voriconazole first-line therapy and discourage the use of liposomal amphotericin B [13]. An earlier guideline established by The European Society of Clinical Microbiology and Infectious Diseases [14] recommends surgical debridement in cases of osteomyelitis due to Scedosporium spp. (strength of recommendation grade A, quality of evidence level IIr). This option was offered to the patient but she and her family declined in favor of conservative management, which was appropriate given her performance status.
In a systematic review of non-Aspergillus mold osteomyelitis cases, the foot was the most commonly involved site (25.3%) [15]. In this review, the median duration of treatment with anti-fungal therapy was 115 days with most patients receiving a combination of surgical debridement and antifungal therapy with good outcomes. There is less data to establish a treatment duration of CNS mold infection. Several months of therapy is likely to be necessary, but the duration of treatment of disseminated scedosporiosis is variable based on clinical response to therapy and net immunosuppression [16].
It is impressive that this high-risk patient survived disseminated S. apiospermum infection, including skin, lung, and brain, despite her early post alloHCT status. Many factors contributed to the successful treatment of her IFD, including early and aggressive treatment with effective anti-mold therapy including voriconazole, rapid engraftment with stable high neutrophil counts, tapering of corticosteroids and optimizing immunosuppression as feasible.
This case demonstrates the debilitating and sometimes devastating nature of IFD and highlights the emergence of lesser-known species that can break through common prophylactic antifungals and mimic common infections. The presence of a positive culture, imaging findings consistent with lung and brain involvement, and positive β-D-Glucan all supported the diagnosis of a disseminated Scedosporium infection. Although we successfully treated IFD in this patient, the best approach is effective prevention. Moreover, this case demonstrates the importance of a thorough “head-to-toe” physical exam in preventing dissemination from “toe to head.”
Availability of data and materials
Not applicable.
Abbreviations
- alloHCT:
-
Allogeneic stem cell transplantation
- AML:
-
Acute myeloid leukemia
- IFD:
-
Invasive fungal disease
- CNS:
-
Central nervous system
- WBC:
-
White blood cells
- ANC:
-
Absolute neutrophil count
- CT:
-
Computerized tomography
- BAL:
-
Bronchioalveolar lavage
- HHV6:
-
Human herpesvirus 6
- EBV:
-
Epstein-Barr virus
- CMV:
-
Cytomegalovirus
- MRI:
-
Magnetic resonance imaging
- CSF:
-
Cerebrospinal fluid
- BID:
-
Twice daily
- MIC:
-
Minimum inhibitory concentration
References
Ustun C, Warlick E, Nathan S, Burns LJ, Weisdorf D. Transplantation provides superior survival high risk myeloid malignancies in older patients. Leuk Lymphoma. 2022;63(10):2494–8. https://doi.org/10.1080/10428194.2022.2076851.
Puerta-Alcalde P, Garcia-Vidal C. Changing Epidemiology of Invasive Fungal Disease in Allogeneic Hematopoietic Stem Cell Transplantation. J Fungi (Basel). 2021;7(10):848. Published 2021 Oct 10. https://doi.org/10.3390/jof7100848.
Husain S, Muñoz P, Forrest G, Alexander BD, Somani J, Brennan K, et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis. 2005;40(1):89–99. https://doi.org/10.1086/426445.
Bupha-Intr O, Butters C, Reynolds G, Kennedy K, Meyer W, Patil S, Bryant P, Morrissey CO, Australasian Antifungal Guidelines Steering Committee. Consensus guidelines for the diagnosis and management of invasive fungal disease due to moulds other than Aspergillus in the haematology/oncology setting, 2021. Intern Med J. 2021;51(Suppl 7):177–219. https://doi.org/10.1111/imj.15592.
Lam K, van Asten S, Nguyen Tea, La Fontaine J, Lavery LA. Diagnostic accuracy of probe to bone to detect osteomyelitis in the diabetic foot: A systematic review. Clin Infect Dis. 2016;63(7):944–8. https://doi.org/10.1093/cid/cid445.
Johnson PW, Collins MS, Wenger DE. Diagnostic utility of T1-weighted MRI characteristics in evaluation of osteomyelitis of the foot. Am J Roentgenol. 2009;192(1):96–100. https://doi.org/10.2214/AJR.08.1376.
Wiederhold NP, Lewis RE. Antifungal activity against Scedosporium species and novel assays to assess antifungal pharmacodynamics against filamentous fungi. Med Mycol. 2009;47(4):422–32. https://doi.org/10.1080/13693780802510224.
Marques DS, Pinho Vaz C, Branca R, Campilho F, Lamelas C, Afonso LP, et al. Rhizomucor and Scedosporium infection post hematopoietic stem-cell transplant. Case Rep Med. 2011;2011:830769.
Cortez KJ, Roilides E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, Knudsen T, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21(1):157–97. https://doi.org/10.1128/CMR.00039-07.
Sydnor MK, Kaushik S, Knight TE Jr, Bridges CL, McCarty JM. Mycotic osteomyelitis due to Scedosporium Apiospermum: MR imaging-pathologic correlation. Skeletal Radiol. 2003;32(11):656–60. https://doi.org/10.1007/s00256-003-0695-0.
Valenzuela Salas I, Martinez Peinada C, Fernandez Meralbell A, Soto Diaz A, Nogueras Morillas P, Martin Castro A, et al. Skin infection caused by Scedosporium apiospermum in immunocompromised patients: report of two cases. Dermatology Online Journal. 2013;19(10).https://doi.org/10.5070/D31910020022.
Odabasi Z, Paetznick V, Rodriguez JR, Chen E, McGinnis MR, Ostrosky-Zeichner L. Differences in beta-glucan levels in culture supernatants of a variety of fungi. Med Mycol. 2006;44(3):267–72. https://doi.org/10.1080/13693780500474327.
Hoenigl M, Salmanton-Garcia J, Walsh TJ, Nucci M, Neoh CF, Jenks JD, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis. 2021;21(8):e246–57. https://doi.org/10.1016/S1473-3099(20)30784-2.
Tortorano AM, Richardson M, Roilides E, van Diepeningen A, Caira M, Munoz P, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. ESCMID and ECMM Publications. 2014;20(3):27–46
Taj-Aldeen SJ, Rammaert B, Gamaletsou M, Sipsas NV, Zeller V, Roilides E, et al. Osteoarticular infections caused by non-Aspergillus filamentous fungi in adult and pediatric patients: a systematic review. Medicine (Baltimore). 2015;94(50):e2078. https://doi.org/10.1097/MD.0000000000002078.
Miceli MH. Central nervous system infections due to Aspergillus and other hyaline molds. J Fungi. 2019;5(3):79. https://doi.org/10.3390/jof5030079.
Acknowledgements
Not applicable.
Funding
There was no funding obtained for the writing of this case report.
Author information
Authors and Affiliations
Contributions
DM wrote the majority of the case report. CU, SN, EB, FG, RL, and AW were major contributors in the writing and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors obtained informed, written consent from the patient to use their data and images in this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marinovic, D.A., Bhaimia, E., Forrest, G.N. et al. Scedosporium infection disseminated “from toe to head” in allogeneic stem cell transplant recipient: a case report. BMC Infect Dis 23, 353 (2023). https://doi.org/10.1186/s12879-023-08345-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08345-2