Abstract
Actinomycosis often leads to cervicofacial infections, but thoracic involvement may also occur. However, the development of empyema is rare. While being followed up with the diagnosis of asthma and bronchiectasis, our case was hospitalized for infected bronchiectasis. As empyema developed in the follow-up, the pleural effusion was drained by tube thoracostomy. Actinomycosis was diagnosed through pleural effusion cytology. Growth of Pseudomonas aeruginosa was observed in sputum culture, and SARS-CoV2 RT-PCR was also positive in nasopharyngeal sampling. Polymicrobial agents can often be detected in actinomycosis. Actinomycosis cases have also been reported in the post-COVID period. Our case is presented since it would be the first in the literature regarding the coexistence of COVID-19, Pseudomonas, and thoracic Actinomycosis (empyema).
Similar content being viewed by others
Background
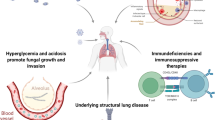
Microorganisms of the genus Actinomyces are gram-positive, anaerobic, non-acid-resistant bacillus determined in the oral flora and cause granulomatous and suppurative infections. While they usually cause cervicofacial infections, thoracic involvement with a rate of 15% may also occur, affecting the lung parenchyma, central airways, pleura, mediastinum, and chest wall [1]. Our case is presented since the coexistence of Coronavirus Disease-19 (COVID-19), Pseudomonas, and thoracic Actinomycosis (empyema) in Actinomyces infections, in which polymicrobial agents are frequently detected, has never been reported before.
Case presentation
A 49-year-old female patient, being followed up with the diagnosis of bronchiectasis, asthma, and scoliosis, had been using inhaled corticosteroid/long-acting beta-2 agonist. The patient did not smoke or drink alcohol, yet her oral hygiene was poor. When she applied to the emergency department due to fever, cough, and increased amount of sputum, her pulse O2 saturation level was 86% (in room air), arterial blood pressure was 118/62 mm Hg, pulse was 86 beats/min, and the temperature was 37.5 °C. On physical examination, there were coarse rales in the left lower zone of the lung. In laboratory tests, pathological values consisted of a C-reactive protein (CRP) of 325 mg/L, a leukocyte count of 25.84 K/uL, and a neutrophil ratio of 95.8%. In the chest computed tomography (CT) imaging, a bronchiectasis image was observed in the middle-lower zone of the left lung (Fig. 1a). Parenteral moxifloxacin treatment was initiated for the patient who was hospitalized. Growth of Pseudomonas aeruginosa was detected in sputum culture, and piperacillin-tazobactam was added to the treatment. In the follow-up, since there was no fever response, increased sputum purulence and amount, decreased respiratory sounds on the left in physical examination, and progression in CRP levels, the patient had another chest X-ray, and total density increase was observed on the left hemithorax (Fig. 1b). Chest CT revealed pleural effusion on the left side. With tube thoracostomy, foul-smelling purulent fluid was drained (Fig. 1d). The biochemistry evaluation of the pleural fluid was compatible with empyema (Table 1). Actinomycosis was diagnosed through pathological examination after dense neutrophils were observed in pleural fluid cytology, sulfur granules in Hematoxylin–Eosin staining (Fig. 2a) and hyphae structures in Giemsa staining (Fig. 2b). There was no growth in the pleural fluid culture. SARS-CoV2 RT-PCR test was also positive in nasopharyngeal sampling. Ampicillin-sulbactam treatment was started. On the 10th day of the tube thoracostomy, the chest tube was removed due to decreased fluid and the radiological response. At discharge, the treatment was continued as amoxicillin + clavulanic acid 2 g/day. At the 2nd-month follow-up, symptoms decreased, and a significant regression was observed in the chest X-ray (Fig. 1c). The patient's treatment still continues.
Discussion
Predisposing factors in actinomycosis infection of endogenous origin include alcoholism, poor oral hygiene, gingival diseases, suppression of the immune system, long-term use of intrauterine devices, and chronic structural respiratory system diseases such as bronchiectasis [1, 2]. Our case was diagnosed with asthma and bronchiectasis and had poor oral hygiene.
Actinomycosis cases usually present with the appearance of consolidation, mass, cavity, or abscess radiologically [3], and pleural effusion rarely develops. In Actinomyces infections, Acinobacillus actinomycetesmcomitans, Eikenella corrodens, Klebsiella spp, Enterobacteriaceae, Fusobacterium spp, Bacteroides spp, Capnocytophagia spp, Staphylococci spp, and Streptococci spp have been isolated, but their role in the pathogenesis remains unclear [4,5,6,7,8,9]. Oxygen deprivation due to polymicrobial infection is likely to increase, facilitating the occurrence of actinomyces infection [4, 6].
In aerobic-based microbial cultures of patients with bronchiectasis, Haemophilus influenza (14–47%), Pseudomonas aeruginosa (5–31%), and Streptococcus pneumonia (2–14%) were reported to be the most frequently isolated pathogens [10,11,12,13]. Although not as much as aerobic agents, Veillonella, Prevotella, and Actinomyces can also be reproduced from anaerobes. Nicotra et al. detected anaerobic organisms in 1.6% of cases with bronchiectasis (10). In healthy non-smokers, the lower respiratory tract is considered sterile. Colonization with potentially pathogenic microorganisms often develops in the presence of chronic bronchitis, chronic obstructive pulmonary disease (COPD), bronchiectasis, bronchial obstruction, and tracheostomy, in which oropharyngeal protection is compromised [14]. Colonization frequency can reach up to the rates of 60–80%. Thus, this condition creates a risk factor for lung infections and causes increased secretion of inflammatory mediators, progressive tissue damage, and increased airway obstruction [15, 16]. Among the colonizing microorganisms, Pseudomonas aeruginosa has a critical place. Pasteur et al. determined P. aeruginosa colonization in bronchiectasis at a rate of 24% [11]. In our case, P. aeruginosa growth was detected in the sputum, which we considered as colonization, and SARS-CoV2 RT-PCR was positive in the nasopharyngeal sampling. In the literature, cases of rhinosinusitis and osteomyelitis due to actinomycosis in the post-COVID period have been reported [17,18,19]. However, our case was the first in the literature due to the coexistence of COVID-19, Pseudomonas, and thoracic actinomycosis. It is also a warning to physicians regarding similar cases that may occur during the COVID-19 pandemic.
Gram staining and histopathological evaluation are more sensitive than culture in diagnosing actinomycosis. The reason for this situation is the slow reproduction of Actinomyces species in an anaerobic culture medium. In addition, previous empirical antibiotic therapy reduces the feasibility of cultures [4, 20].
In our case, there was no growth in the pleural fluid culture. The diagnosis was made by the presence of sulfur granules and hyphae structures in cytology.
Although the treatment regimen and duration are unclear, beta-lactams such as penicillin G, amoxicillin, ceftriaxone, doxycycline, and clindamycin, along with erythromycin for pregnant women, are recommended. The duration of treatment is recommended as 6–12 months [1]. Surgery is especially useful in the presence of an abscess, empyema, and massive hemoptysis (5,6). In our patient, good treatment response was observed in the 2nd-month to oral amoxicillin-clavulanic acid treatment, and the patient's treatment still continues.
Conclusion
Polymicrobial infection factors should be considered in patients with chronic structural lung diseases. It is crucial to perform a cytological examination of the materials in the absence of growth in cultures and include diseases such as actinomycosis in the differential diagnosis.
Availability of data and materials
Not applicable.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2 RT-PCR:
-
Severe Acute Respiratory Syndrome Coronavirus- 2 Reverse Transcriptase Polymerase Chain Reaction
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- COPD:
-
Chronic obstructive pulmonary disease
- H&E:
-
Hematoxylin–Eosin
References
Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J. 2003;21(3):545–51. https://doi.org/10.1183/09031936.03.00089103.
Mutlu P, Mirici NA, Güven MI. Primary Pulmonary Actinomycosis Mimicking Malignancy. Med Bull Haseki Pub Galenos Publ. 2018;56:74–7.
Kolditz M, Bickhardt J, Matthiessen W, Holotiuk O, Höffken G, Koschel D. Medical management of pulmonary actinomycosis: data from 49 consecutive cases. J Antimicrob Chemother. 2009;63(4):839–41. https://doi.org/10.1093/jac/dkp016.
Grzywa-Celińska A, Emeryk-Maksymiuk J, Szmygin-Milanowska K, Czekajska-Chehab E, Milanowski J. Pulmonary actinomycosis - the great imitator. Ann Agric Environ Med. 2017;25(2):211–2. https://doi.org/10.26444/aaem/75652.
Farrokh D, Rezaitalab F, Bakhshoudeh B. Pulmonary actinomycosis with endobronchial involvement: a case report and literature review. Tanaffos. 2014;13(1):52–6 PMID: 25191495.
Skehan N, Naeem M, Reddy RV. Endobronchial actinomycosis: successful treatment with oral antibiotics. BMJ Case Rep. 2015;2015(17):bcr2015212754. https://doi.org/10.1136/bcr-2015-212754.
Valour F, Sénéchal A, Dupieux C, Karsenty J, Lustig S, Breton P, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;5(7):183–97. https://doi.org/10.2147/IDR.S39601.
Nagaoka K, Izumikawa K, Yamamoto Y, Yanagihara K, Ohkusu K, Kohno S. Multiple lung abscesses caused by Actinomyces graevenitzii mimicking acute pulmonary coccidioidomycosis. J Clin Microbiol. 2012;50(9):3125–8. https://doi.org/10.1128/JCM.00761-12.
Jbeli A, Niccum DE, Erickson, S. A Case of Actinomyces Neuii Empyema and Association with Non-Small Cell Lung Carcinoma. In: TP99. TP099 Curıous Mycobacterıal Cases And Mımıckers. Am J Respir Crit Care Med. 2021;203:A4063. https://www.atsjournals.org.
Nicotra MB, Rivera M, Dale AM, Shepherd R, Carter R. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest. 1995;108(4):955–61. https://doi.org/10.1378/chest.108.4.955.
Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1277–84. https://doi.org/10.1164/ajrccm.162.4.9906120.
Angrill J, Agustí C, de Celis R, Rañó A, Gonzalez J, Solé T, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57(1):15–9. https://doi.org/10.1136/thorax.57.1.15.
King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101(8):1633–8. https://doi.org/10.1016/j.rmed.2007.03.009.
Cabello H, Torres A, Celis R, El-Ebiary M, de la BellacasaPuig J, Xaubet A, et al. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: a bronchoscopic study. Eur Respir J. 1997;10(5):1137–44. https://doi.org/10.1183/09031936.97.10051137.
Angrill J, Agustí C, Torres A. Bronchiectasis. Curr Opin Infect Dis. 2001;14(2):193–7. https://doi.org/10.1097/00001432-200104000-00014.
Pang JA, Cheng A, Chan HS, Poon D, French G. The bacteriology of bronchiectasis in Hong Kong investigated by protected catheter brush and bronchoalveolar lavage. Am Rev Respir Dis. 1989;139(1):14–7. https://doi.org/10.1164/ajrccm/139.1.14.
Jagtap SV, Hulwan A, Vartak S, Singh R, Jagtap SS. Co-infection of mucormycosis and actinomycosis in COVID-19 infection. Int J Health Sci Res. 2021;11:127–30.
Arshad W, Mahmood Kamal M, Rafique Z, Rahat M, Mumtaz H. Case of maxillary actinomycotic osteomyelitis, a rare post COVID complication-case report. Ann Med Surg. 2022;80:104242. https://doi.org/10.1016/j.amsu.2022.104242.
Jawanda MK, Narula R, Gupta S, Sharma V, Sidhu SK, et al. Mixed Infections (Mucormycosis, Actinomycosis and Candidiasis) Leading to Maxillary Osteomyelitis in a Diabetic Mellitus Patient in Post COVID Phase: First Case Report. Acta Medica (Hradec Kralove). 2021;64(4):218–23. https://doi.org/10.14712/18059694.2022.5.
Jung HW, Cho CR, Ryoo JY, Lee HK, Ha SY, Choi JH, et al. Actinomyces meyeri Empyema: A Case Report and Review of the Literature. Case Rep Infect Dis. 2015;2015:291838. https://doi.org/10.1155/2015/291838.
Acknowledgements
Not applicable.
Funding
The study received no external funding.
Author information
Authors and Affiliations
Contributions
EA wrote the initial draft of manuscript. FK, HO, MYH managed the diagnosis and treatment. EA, FK, HO, MYH approved the fnal version of the manuscript and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written and verbal informed consent was obtained from the patient for publication of this case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Afsin, E., Kucuk, F., Ozcelik, H. et al. Coexistence of COVID-19, Pseudomonas, and thoracic actinomycosis in a cystic bronchiectasis case. BMC Infect Dis 23, 203 (2023). https://doi.org/10.1186/s12879-023-08215-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08215-x