Abstract
Background
Ureaplasma urealyticum is the most prevalent genital mycoplasma isolated from the urogenital tract of females, but there is no unified treatment plan. This study aimed to evaluate the efficacy of azithromycin in treating Ureaplasma urealyticum.
Methods
From the earliest to June 2022, published randomized controlled trials (RCTs) on azithromycin treatment of Ureaplasma urealyticum were retrieved by searching PubMed, Embase, Cochrane Library, and Web of Science. Two reviewers independently extracted the data. We utilized the Cochrane risk-of-bias assessment technique to assess the quality of included RCTs. The data were analyzed using the R language (version 4.0.4) software.
Results
Seven RCTs were finally included, involving 512 participants (240 in the experimental group, 272 in the control group). The experimental group was treated with azithromycin monotherapy, while the control group was treated with doxycycline or a placebo. Meta-analysis results suggested that azithromycin has a comparable therapeutic effect on Ureaplasma urealyticum in comparison to that of controls (risk ratio [RR] = 1.03, 95% confidence interval [CI] 0.94–1.12). Subgroup analysis showed that the dose and duration of azithromycin may don’t affect its efficacy.
Conclusion
Regarding the meta-analysis that we performed based on existing clinical studies, azithromycin is quite effective in treating Ureaplasma urealyticum.
Similar content being viewed by others
Background
Ureaplasma species are the most prevalent genital mycoplasma isolated from the urogenital tract of both men and women [1]. Ureaplasma has 14 known serotypes and is divided into two biovars, Ureaplasma parvum and Ureaplasma urealyticum [2]. Currently, commonly used drugs in Ureaplasma urealyticum treatment include quinolones, macrolides and tetracyclines. The growth in the resistance rate of fluoroquinolones, as the first-line clinical drugs, is particularly obvious. Resistance to macrolides and tetracyclines has been reported [3,4,5]. Azithromycin exhibits high potency against clinical ureaplasma isolates in vitro [6]. However, the clinical efficacy of azithromycin in treating Ureaplasma urealyticum remains controversial; therefore, it is necessary to conduct a rigorous systematic evaluation of existing research literature to provide more reliable evidence-based medicine for disease prevention and treatment. It would provide an objective reference for the clinical rationale and scientific use of antimicrobial drugs and the control of Ureaplasma urealyticum resistance.
Methods
This systematic review was registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) database (registration No. INPLASY202240001).
Search strategy
The study was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [7]. A literature search on PubMed, Embase, Web of Science, and Cochrane Library was conducted, and we limited our search to high-quality studies published until June 2022. The search was undertaken with Medical Subject Headings (MeSH), and appropriate adjustments were made according to the different databases. Search terms included: (1) Ureaplasma urealyticum or Ureaplasma urealyticum biovar 2, (2) Azithromycin or Azythromycin or Sumamed or Toraseptol or Vinzam or CP-62,993 or CP 62,993 or CP62993 or Zithromax or Azitrocin or Azadose or Ultreon or Zitromax or Azithromycin Dihydrate or Dihydrate, Azithromycin or Azithromycin Monohydrate or Monohydrate, Azithromycin or Goxal or Zentavion. The retrieval search strategy is shown in Additional file 1. The reference lists of the articles identified in the primary search or the relevant reviews identified using the search were also reviewed by three independent reviewers.
Inclusion/exclusion criteria
The inclusion criteria were as follows: RCT study of azithromycin in treating Ureaplasma urealyticum infection in a female reproductive tract over 18 years of age. The exclusion criteria were as follows: (1) letters, reviews, guidelines or editorials; (2) animal studies or cell studies; (3) studies only including the use of azithromycin in males and infants; (4) non-randomized controlled studies; (5) studies in which treatment effect values could not be obtained.
Data extraction and quality assessment
The following information was extracted by two independent reviewers (Jinyu Wang and Qisheng Wang): author(s), publication year, research design, participant number, types of antibiotics, treatment dose, and mycoplasma cure. Any disagreement on specific studies between the two reviewers was resolved through discussion or consultation with the third reviewer (Zuyu Liang). The quality of the studies was established according to the Newcastle–Ottawa Scale (NOS).
Risk of bias assessment
Egger’s test [8] was used to exam the publication bias in included study and the revised Cochrane risk of bias tool for randomized trials (RoB2) [9] was applied to assess the risk of bias of RCTs. All risk of bias assessment was assessed by two authors (Weibin Fan and Qisheng Wang) individually and any disagreement was resolved by a third author (Lin Zhang).
Statistical analysis
The R language (version 4.0.4) software was used for data analysis and visualization. specifically, the meta and metafor packages were used for data processing and forest and funnel plots. Dichotomous variables were analyzed using the odds ratio (OR) and its 95% confidence interval (CI) as statistics for efficacy analysis. Heterogeneity was tested using a Chi-squared test (α = 0.1), combined with a Q test and I2 test, and if P > 0.1 and I2 ≤ 50%, which suggested less heterogeneity between the studies, a fixed-effect model was used. Otherwise, a random-effect model was used. Significant heterogeneity was addressed via subgroup analysis or sensitivity analysis.
Results
Study selection and characteristics
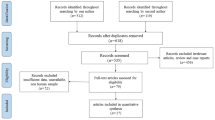
Three researchers searched PubMed, Embase, Web of Science, and Cochrane Library and found 83 articles on PubMed, 20 articles on Embase, 44 articles on Web of Science, and 3 articles on Cochrane Library. The search strategy and selection are shown in Fig. 1. Finally, seven studies were included for meta-analysis from the 130 initial research studies after the exclusion strategy. The characteristics of the selected studies are shown in Table 1. These studies included 512 patients with Ureaplasma urealyticum (240 patients treated with azithromycin and 272 patients as the control).
Efficacy of azithromycin treatment
The primary outcome suggested no superiority in treating Ureaplasma urealyticum in the female reproductive tract with other antibiotics. Compared to other antibiotics, azithromycin has a comparable therapeutic effect on Ureaplasma urealyticum infection. The fixed effect of RR was 1.03 [0.94, 1.12], while the random effect of RR was 0.98 [0.80, 1.20] (Fig. 2).
Subgroup analysis
Subgroup analysis was performed according to the therapy drug in control group and dose and duration of azithromycin. When we divided the included RCTs into subgroups according to the drug prescribed in control group, we came to similar conclusions. Whether the control group was treated with doxycycline or traditional Chinese medicine, azithromycin had a similar efficacy. The fixed effect of RR was 1.04 [0.95, 1.13] (Fig. 3). We also considered the efficacy of azithromycin on Ureaplasma urealyticum at different doses and duration of administration, meta-analyses of the subgroup showed that they had a comparable therapeutic effect compared to controls, whether given as a single dose of 1 g or 0.5 g QD for 7 days. The fixed effect of RR in subgroup of a single dose of 1 g was 0.99 [0.88, 1.12] and in subgroup of 0.5 g QD for 7 days was 1.02 [0.87, 1.20] (Fig. 4).
Quality assessment and risk of bias
Publication bias was tested for azithromycin treatment efficiency, as displayed in Fig. 5, and the funnel plot demonstrated good symmetry. Egger’s test showed no obvious publication bias in the rate of azithromycin treatment efficiency (p = 0.297). Risk of bias in randomized trials was examined with the Cochrane Risk of Bias 2 (RoB2) tool. According to RoB2, two studies were rated as having a high risk of bias. Other five studies were rated as having a low risk of bias. The detailed results of the risk of bias analyses are available in Figs. 6 and 7.
Discussion
Recently, the prevalence of cervical mycoplasma infection has been increasing yearly [15, 16]. Mycoplasma cervicalis infection can cause a series of complications, including infertility, spontaneous abortion, pelvic inflammatory disease, and ectopic pregnancy, posing a great threat to women’s health during their reproductive years. In addition, habitual abortion, frequent sexual intercourse, and childbirth may also damage the cervix, causing pathogens to invade and form inflammation and, ultimately, induce cervicitis [6, 17]. Mycoplasma is the main pathogen that causes cervicitis and does not usually invade the bloodstream but rather often binds to the epithelial cells of the genitourinary tract or respiratory tract through adhesion and damages the cells via different mechanisms of action [18]. Some studies [16, 19] have reported that Ureaplasma urealyticum infection is the most prevalent in patients with cervicitis, followed by Ureaplasma urealyticum + mycoplasma hominis infection, whose infection rate is the lowest in mycoplasma hominis. The reason for this may be that Ureaplasma urealyticum is by far the simplest and smallest cell with the ability to reproduce itself, making it easier to invade a damaged cervix and cause infection compared to other pathogens [20]. Mycoplasma has no cell wall; thus, antibacterial drugs used for a cell wall are ineffective in its treatment.
At this stage, clinical treatment of mycoplasma is based on drugs such as quinolones, tetracyclines, macrolides, etc. The sensitivity of different mycoplasmas to different drugs is not exactly the same, and in vitro drug sensitivity tests also often have poor clinical application; therefore, the choice of antibiotics has not formed the first-line recommendation. In previous studies, it has been clearly stated that quinolones should not be used for empirical treatment of Ureaplasma urealyticum infection. Due to the high frequency of clinical use of ciprofloxacin and levofloxacin in recent years, Ureaplasma urealyticum is prone to developing resistance to them [21]. The resistance mechanism is mainly manifested by mutations in chromosomal DNA pro-rotase and topoisomerase IV, which are maintained in a highly resistant, stable state. Antibiotics containing β-lactamases are also ineffective against Ureaplasma urealyticum infection because they have no cell wall structure.
Azithromycin, a macrolide that interferes with cellular protein synthesis, is often used to treat patients with mycoplasma cervicitis. Azithromycin is well absorbed orally and has a wide tissue distribution. When azithromycin enters the body, the active drug is transported by macrophages to the site of infection and acts to inhibit anaerobic bacteria and mycoplasma for a long time. In addition, as far as drug safety is concerned, according to the classification of the drug look-up table for pregnancy risk class issued by the FDA, macrolides belong to class B. They provide better therapeutic safety for women of childbearing age and during pregnancy, as well as avoiding adverse pregnancy outcomes that should be caused by drug treatment [22].
This meta-analysis demonstrated that azithromycin has a positive therapeutic effect on Ureaplasma urealyticum infection and there is no correlation with the dose of azithromycin, whether it is a single dose of 1 g or 0.5 g QD for 6 days. Compared with the control group, two subgroups both showed comparable efficacy. However, a meta-analysis of the safety of azithromycin for Ureaplasma infection could not be conducted due to the limited amount of relevant literature as well as the lack of safety studies in some of the included studies. Previous research has generally indicated that azithromycin has a favorable safety profile in various treatment situations.
The present study followed PRISMA guidelines to ensure the quality of its methodology. The reliability of study results was also assessed using GRADE grading method. However, there are limitations to this study that should be identified. These include a small amount of included literature and insufficient sample size, the potential for language bias due to the inclusion of only English literature in the search strategy, and the need for larger, multi-regional, multi-center, randomized, double-blind clinical trials to validate the findings.
Conclusion
Regarding the meta-analysis that we performed based on existing clinical studies, azithromycin has a comparable therapeutic effect on Ureaplasma urealyticum infection in the female reproductive tract compared to other antibiotics.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RCTs:
-
Randomized controlled trials
- INPLASY:
-
International Platform of Registered Systematic Review and Meta-analysis Protocols
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- MeSH:
-
Medical Subject Headings
- NOS:
-
Newcastle–Ottawa Scale
- OR:
-
Odds ratio
- CI:
-
95% Confidence interval
References
Strauss M, Colodner R, Sagas D, Adawi A, Edelstein H, Chazan B. Detection of Ureaplasma Species by a semi-quantitative PCR test in urine samples: can it predict clinical significance? Isr Med Assoc J IMAJ. 2018;20(1):9–13.
Pitcher D, Sillis M, Robertson JA. Simple method for determining biovar and serovar types of Ureaplasma urealyticum clinical isolates using PCR-single-strand conformation polymorphism analysis. J Clin Microbiol. 2001;39(5):1840–4.
Sprong KE, Mabenge M, Wright CA, Govender S. Ureaplasma species and preterm birth: current perspectives. Crit Rev Microbiol. 2020;46(2):169–81.
Beeton ML, Chalker VJ, Jones LC, Maxwell NC, Spiller OB. Antibiotic resistance among clinical Ureaplasma isolates recovered from Neonates in England and Wales between 2007 and 2013. Antimicrob Agents Chemother. 2016;60(1):52–6.
Boujemaa S, Mlik B, Ben Allaya A, Mardassi H, Ben Abdelmoumen Mardassi B. Spread of multidrug resistance among Ureaplasma serovars, Tunisia. Antimicrob Resist Infect control. 2020;9(1):19.
ACOG Practice Bulletin No. 142: Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014;123(2 Pt 1):372–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res Ed). 2021;372:n71.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed). 1997;315(7109):629–34.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed). 2019;366:l4898.
Liu WE, Tan ZY, Xia RY, Zou ZX, Gao WH, Kuang JL, Ding LL. Treatment of Ureaplasma urealyticum infection patients of Qi deficiency blood stasis syndrome by pengyan pill: a clinical observation. Chinese J Integr Tradit West Med. 2013;33(5):590–3.
Skerk V, Schönwald S, Strapac Z, Beus A, Francetić I, Krhen I, Lesko V, Vukovic J. Duration of clinical symptoms in female patients with acute urethral syndrome caused by Ureaplasma urealyticum treated with azithromycin or doxycycline. J Chemother. 2000;12(6):533–5.
Skerk V, Barsić B, Car V, Schönwald S, Klinar I. Comparative analysis of azithromycin and doxycycline efficacy in the treatment of female patients with acute urethral syndrome caused by Ureaplasma urealyticum. J Chemother. 2000;12(2):186–8.
Sendağ F, Terek C, Tuncay G, Ozkinay E, Güven M. Single dose oral azithromycin versus seven day doxycycline in the treatment of non-gonococcal mucopurulent endocervicitis. Aust N Z J Obstet Gynaecol. 2000;40(1):44–7.
Steingrimsson O, Olafsson JH, Thorarinsson H, Ryan RW, Johnson RB, Tilton RC. Azithromycin in the treatment of sexually transmitted disease. J Antimicrob Chemother. 1990;25(Suppl A):109–14.
Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol JEADV. 2016;30(10):1650–6.
Leli C, Mencacci A, Latino MA, Clerici P, Rassu M, Perito S, Castronari R, Pistoni E, Luciano E, DeMaria D, et al. Prevalence of cervical colonization by Ureaplasma parvum, Ureaplasma urealyticum, Mycoplasma hominis and Mycoplasma genitalium in childbearing age women by a commercially available multiplex real-time PCR: an Italian observational multicentre study. J Microbiol Immunol Infect. 2018;51(2):220–5.
Oh KJ, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS, Yoon BH. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstetr Gynecol. 2019;221(2):140.e141-140.e118.
Baumann L, Cina M, Egli-Gany D, Goutaki M, Halbeisen FS, Lohrer GR, Ali H, Scott P, Low N. Prevalence of Mycoplasma genitalium in different population groups: systematic review andmeta-analysis. Sex Transm Infect. 2018;94(4):255–62.
Esen B, Gozalan A, Sevindi DF, Demirbas A, Onde U, Erkayran U, Karakoc AE, Hasçiçek AM, Ergün Y, Adiloglu AK. Ureaplasma urealyticum: presence among sexually transmitted diseases. Jpn J Infect Dis. 2017;70(1):75–9.
Pollack JD. The necessity of combining genomic and enzymatic data to infer metabolic function and pathways in the smallest bacteria: amino acid, purine and pyrimidine metabolism in Mollicutes. Front Biosci J Virtual Libr. 2002;7:d1762-1781.
Jensen JS, Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol JEADV. 2022;36(5):641–50.
Taylor-Cousar JL, Jain R, Kazmerski TM, Aitken ML, West NE, Wilson A, Middleton PG, Nash EF. Concerns regarding the safety of azithromycin in pregnancy-relevance for women with cystic fibrosis. J Cyst fibrosis: official J Eur Cyst Fibros Soc. 2021;20(3):395–6.
Acknowledgements
The authors thank all the participants and their physicians for their contributions to this study.
Funding
The study had not accepted any fundings’ support.
Author information
Authors and Affiliations
Contributions
WF, QW and LZ conceived the study and its design. QW, JW and ZL helped in study search and selection. WF and QW finished data extraction and quality assessment. LZ helped in Statistical analysis. WF, QW and LZ drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fan, W., Wang, Q., Liang, Z. et al. Efficacy of azithromycin in treating Ureaplasma urealyticum: a systematic review and meta-analysis. BMC Infect Dis 23, 163 (2023). https://doi.org/10.1186/s12879-023-08102-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08102-5