Abstract
Background
The effect of angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) on mortality was preliminarily explored through the comparison of ACEIs/ARBs with non-ACEIs/ARBs in patients with coronavirus disease 2019 (COVID-19). Reaching a conclusion on whether previous ACEI/ARB treatment should be continued in view of the different ACE2 levels in the comparison groups was not unimpeachable. Therefore, this study aimed to further elucidate the effect of ACEI/ARB continuation on hospital mortality, intensive care unit (ICU) admission, and invasive mechanical ventilation (IMV) in the same patient population.
Methods
We searched PubMed, the Cochrane Library, Ovid, and Embase for relevant articles published between December 1, 2019 and April 30, 2022. Continuation of ACEI/ARB use after hospitalization due to COVID-19 was considered as an exposure and discontinuation of ACEI/ARB considered as a control. The primary outcome was hospital mortality, and the secondary outcomes included 30-day mortality, rate of ICU admission, IMV, and other clinical outcomes.
Results
Seven observational studies and four randomized controlled trials involving 2823 patients were included. The pooled hospital mortality in the continuation group (13.04%, 158/1212) was significantly lower than that (22.15%, 278/1255) in the discontinuation group (risk ratio [RR] = 0.45; 95% confidence interval [CI], 0.28–0.72; P = 0.001). Continuation of ACEI/ARB use was associated with lower rates of ICU admission (10.5% versus 16.2%, RR = 0.63; 95% CI 0.5–0.79; P < 0.0001) and IMV (8.2% versus 12.5%, RR = 0.62; 95% CI 0.46–0.83, P = 0.001). Nevertheless, the effect was mainly demonstrated in the observational study subgroup (P < 0.05). Continuing ACEI/ARB had no significant effect on 30-day mortality (P = 0.34), acute myocardial infarction (P = 0.08), heart failure (P = 0.82), and acute kidney injury after hospitalization (P = 0.98).
Conclusion
Previous ACEI/ARB treatment could be continued since it was associated with lower hospital deaths, ICU admission, and IMV in patients with COVID-19, although the benefits of continuing use were mainly shown in observational studies. More evidence from multicenter RCTs are still needed to increase the robustness of the data.
Trial registration PROSPERO (CRD42022341169). Registered 27 June 2022
Similar content being viewed by others
Background
The coronavirus disease 2019 (COVID-19) pandemic has imposed unprecedented challenges on both the worldwide health system and global economic development [1]. COVID-19 is a viral multiorgan disease although it predominantly originates from the respiratory system [2]. In particular, the renin–angiotensin–aldosterone system (RAAS) has been implicated, as it has not only been associated with the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) through angiotensin-converting enzyme 2 (ACE2) [3], but also with inflammatory lung injury. During the development of COVID-19, RAAS plays multiple roles. On one hand, angiotensin-converting enzyme (ACE) converts angiotensin I (Ang I) into angiotensin II (Ang II), increases oxidative stress, promotes inflammation, and induces fibrosis through the type 1 angiotensin receptor (AT1R) [4, 5]. On the other hand, ACE2, another enzyme in the RAAS, transforms Ang II into Ang-(1–7), increases the level of nitric oxide, and alleviates inflammation by combining with the Mas receptor [6, 7]. In theory, ACE inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) potentially relieve SARS-CoV-2-induced lung injury by downregulating the proinflammatory effect of the ACE-Ang II-AT1R pathway, thus redirecting considerable attention to the potential benefit of ACEIs/ARBs [8, 9]. Nonetheless, animal model studies have also indicated that ACEIs/ARBs could upregulate the expression and enhance the activity of ACE2 [10, 11], which plays an important role in the mechanism by which SARS-CoV-2 enters the human lungs [12, 13], and thereby potentially aggravates lung injury. As a result, there are two opposing hypotheses regarding the effect of ACEIs/ARBs on SARS-CoV-2-induced lung injury, that is, whether ACEIs/ARBs are protective or harmful [7, 14].
The results of certain clinical trials comparing patients taking ACEIs/ARBs with those receiving other antihypertensive agents revealed that ACEIs/ARBs were associated with a lower risk of mortality in patients with COVID‐19 and hypertension [15]; however, other studies found that ACEIs/ARBs did not affect mortality or severe diseases in a conservative tone [16,17,18,19]. In these studies, the levels of ACE2 in COVID-19 patients with and without the chronic use of ACEIs/ARBs were not completely identical; the expression of ACE2 in the former might have been regulated by the chronic use ACEIs/ARBs nevertheless, ACE2 in the latter was in the protoform state. In other words, there are two differences existed between the comparative groups: the intervention strategy (taking ACEIs/ARBs or not) and baseline ACE2 levels. Hence, an immediate conclusion regarding the continuation of ACEIs/ARBs in patients who have previously undergone chronic ACEI/ARB treatment may be premature, according to the aforementioned studies. Therefore, comparison of mortality between patients who continue and those who discontinue previous ACEI/ARB treatment is warranted [7]. Such a comparison potentially contributes to the elucidation of the feasibility of continued ACEI/ARB after a COVID-19 diagnosis [20,21,22]. This study aimed to further determine the effect of ACEI/ARB continuation on hospital mortality, ICU admission, and IMV compared with discontinuation of ACEI/ARB in the patients with the chronic use of ACEIs/ARBs before being infected with SARS-CoV-2.
Methods
General information
This systematic review, meta-analysis, and meta-regression analysis was performed under the guidance of the PRISMA statement [23]. The protocol was registered in the PROSPERO database (CRD42022341169). Ethical review and informed consent were waived for this type of study. In this study, participants comprised adult patients with COVID-19 who had a history of chronic ACEI/ARB therapy for the treatment of their underlying diseases. We considered the continuation of ACEIs/ARBs after hospitalization for COVID-19 as an exposure and the discontinuation of ACEIs/ARBs as a control. The primary outcome of interest was hospital mortality, and the secondary outcomes were 30-day mortality, rate of intensive care unit (ICU) admission, rate of invasive mechanical ventilation (IMV) use, acute myocardial infarction, new or worsening congestive heart failure, and new onset acute kidney injury after hospitalization.
Search strategy
The electronic search was performed in PubMed, the Cochrane Library, and Ovid Embase for relevant articles published between December 1, 2019 and April 30, 2022. The following combinations of terms or keywords were used: (COVID-19 OR corona virus OR SARS-CoV-2) AND (ACE inhibitors OR ARB OR angiotensin-converting enzyme inhibitors OR angiotensin receptor blockers OR RAS inhibitors OR renin–angiotensin system inhibitors). We did not stipulate any further restrictions except the publication time. Duplicate papers were identified and counted once. The references of the relevant publications were verified manually to identify potentially eligible studies.
Eligibility criteria
Full texts were evaluated for potentially eligible studies based on the inclusion and exclusion criteria by Q.L. and W.F. independently. In case of disagreement, they discussed and consulted a third author (C.Z.) for arbitration. The inclusion criteria were as follows: (1) participants aged ≥ 18 years, (2) COVID-19 hospitalization and diagnosis, (3) ACEI/ARB administration as a chronic treatment for any indication prior to COVID-19 infection, (4) at least one objective to compare the effect of continuing previously administered ACEIs/ARBs with that of discontinuing ACEIs/ARBs, and (5) at least one outcome of interest extractable from the publication and Additional file 1. The exclusion criteria included the following: (1) patients aged < 18 years, (2) trials comparing effect of ACEIs/ARBs with that of placebos or other antihypertensive agents, (3) sole availability of an abstract or meeting paper without published full text, and (4) non-original studies, such as editorials, case reports, reviews, and guidelines. Additionally, only one of the papers was included if multiple articles were published for the same trial.
Data extraction and quality assessment of the included studies
The characteristics and outcomes of interest of the included studies were extracted and managed using a spreadsheet. The numbers of events, and the sample sizes in each group were directly extracted to acquire dichotomous data from the articles and appendixes. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of cohort studies in this meta-analysis [24]. The NOS was used to evaluate quality with regard to three aspects: selection, comparability, and outcome. One star was awarded for each numbered item within the selection and outcome categories if it qualified, while a maximum of two stars was awarded for comparability. A maximum of nine stars could be awarded to a study. For randomized controlled trials (RCTs), the Cochrane Collaboration tool was adopted to assess the risk of bias [25]. This tool appraised the quality in six dimensions: sequence generation, allocation concealment, blindness to participants and personnel, outcome assessment, data integrality of the outcome, and selective reporting. Each item was classified as follows: low risk, high risk, or unclear risk of bias based on the related information. Two authors (Q.L. and W.F.) performed the evaluations independently. Disagreements were resolved by discussion and subsequently referred to a third author (C.Z.) for arbitration.
Statistical analysis
We adopted risk ratios (RRs) with 95% confidence intervals (CIs) to determine the effect size of dichotomous outcomes in this study. The pooled RR was calculated using the Mantel–Haenszel method with the fixed model if the heterogeneity was not high (< 75%); otherwise, the random model was employed. Additionally, to get the adjusted RR and evaluate the stability of the result, the RRs were logarithmically transformed and then pooled together combined with the corresponding stand errors, which were calculated from 95% CIs of crude RR. Heterogeneity was estimated quantitatively using I square (I2) [26, 27], and the value of I2 was divided into three levels: < 50% indicated low heterogeneity, 50%-75% moderate heterogeneity, and ≥ 75% high heterogeneity [28]. Subgroup analyses were performed based on the research design (RCT or observational study) and number of research centers (single or multiple-centers). Sensitivity analyses were performed by removing each study one by one or changing the effect model to observe the changes in heterogeneity and check whether the results remained stable, especially after removing some low-quality studies. Univariate and multivariate meta-regression analyses based on random-effects restricted maximum likelihood were performed to estimate the effect of the characteristics of trials on the relationship between ACEIs/ARBs and hospital mortality. P < 0.05 was considered as a significant difference. We utilized both Stata/IC (version 16.1, Single-user License, StataCorp, Texas, USA) and Review Manager 5.3.5 (Cochrane Collaboration, Oxford, UK) for statistical analyses.
Results
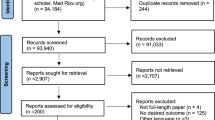
As shown in Fig. 1, 2236 articles were identified from the electronic search. Thereafter, 2160 were excluded based on their titles and abstracts. Further full text evaluation eliminated 65 studies, including one study that reported overlapping data from a different perspective [29]. Finally, we included 11 studies, including seven observational studies [30,31,32,33,34,35,36] and four RCTs [37,38,39,40]. In total, 2823 hospitalized patients were recruited in the eleven studies. Five studies were performed in single centers and six in multiple centers. The studies were distributed across several countries, including Canada [37], United States [31, 32], Spain [35, 36], Italy [30], Brazil [40], Iran [33], France [34], Austria, and Germany [38]. One study simultaneously covered several American countries and Sweden [39]. More characteristics of the included studies are presented in Table 1. The reasons of discontinuing ACEI/ARB were summarized in Additional file 1: Table S1.
Quality assessment of the included studies
The quality of the included trials was high in both RCTs and cohort studies. The bias in RCTs was rated as low risk for most of the appraisal items, while high risk of bias or unclear risk of bias was predominantly associated with the course of performance, that is, participants and personnel were not blinded for the safety of the patients (Table 2). All the included cohort studies were awarded at least five stars, and half of them were awarded eight stars, thus yielding a high quality of cohort studies (Table 3). In the cohort studies, all the patients were confirmed using clear diagnostic criteria, and they originated from the same population. Simultaneously, the continuation or discontinuation of ACIEs/ARBs was described explicitly or judged according to the electronic medical record system, and stars were awarded for the selected items and for comparability among all the included studies. Outcome assessment was awarded a star if the mortality was extracted from the record system regardless of blinding. Follow-up information was considered not to qualify for a star if information about it could not be located in the article.
Effect of ACEI/ARB continuation on hospitalization and 30-day mortality
Nine studies covering 2467 patients, reported hospital mortality, and the pooled hospital mortality was 13.04% (158/1212) and 22.15% (278/1255) in the continuation group and discontinuation group respectively, and the difference was significant in random effect model (RR = 0.45; 95% CI 0.28–0.72; z = 3.34, P = 0.0008) with high heterogeneity among studies (I2 = 80%; P < 0.00001) (Fig. 2A),sensitivity analysis demonstrated that the difference was still significant in fixed effect model (RR = 0.60; 95% CI 0.51–0.71; z = 5.83, P < 0.00001). Five studies, recruiting 1178 patients, reported on 30-day mortality, and according to the pooled results, no significant difference was observed between the continuation and discontinuation of ACEIs/ARBs (6.8% versus 7.5%; RR, 0.83; 95% CI 0.56–1.22; z = 0.96; I2 = 51%; P = 0.34) (Fig. 2B).
Effect of ACEI/ARB continuation on ICU admission and IMV use
As shown in Fig. 3A, the pooled results indicate that the ICU admission rate was lower in the ACEI/ARB continuation group (10.5%, 102/927) than in the ACEI/ARB discontinuation group (16.2%, 153/942), and the difference between the two groups was statistically significant (RR = 0.63; 95% CI 0.5–0.79; z = 3.94; P < 0.0001) with moderate heterogeneity (I2 = 51%; P = 0.05). Moreover, the continuation of ACEIs/ARBs also was associated with the lower rate of IMV (8.2% versus 12.5%; RR = 0.62; 95% CI 0.46–0.83; P = 0.001) with low heterogeneity (I2 = 43%; P = 0.11) (Fig. 3B).
Effect of ACEI/ARB continuation on key organs
A few included studies reported the outcomes of key organs. The pooled results indicated that ACEI/ARB continuation did not have significant effects on acute myocardial infarction, new or worsening congestive heart failure, and new onset acute kidney injury (P = 0.08, P = 0.82, and P = 0.98, respectively, Fig. 4).
Subgroup and sensitivity analysis
Subgroup analysis based on the research design (RCT or observational study) revealed a significant effect of ACEI/ARB continuation, predominantly in observational studies (RR 0.42; 95% CI 0.25–0.71; P = 0.001), and the pooled in-hospital mortality was higher in observational studies than in RCTs in both the intervention (17.4% versus 2.3%) and control (29.7% versus 3.1%) groups. The benefit in reducing ICU admission and IMV use mainly derives from observational studies (P < 0.05). Subgroup analysis based on the number of research centers (single or multiple centers) revealed that the benefit of ACEI/ARB continuation was predominantly observed in single-center studies with low heterogeneity (RR 0.20; 95% CI 0.28–0.39; P < 0.00001; I2 = 0%) (Additional file 1: Fig. S1). According to the description about blood pressure (Additional file 1: Table S1), we allocated the studies into three categories: studies in which patients had comparable blood pressure between the two groups, studies in which some of the patients in the discontinuing group had hypotension, and studies in which patients had unstated blood pressure (Additional file 1: Table S2). Benefit in hospital mortality, rate of ICU admission, and IMV use was found in the studies in which some of the patients in the discontinuing group had hypotension (Table 4). Information about AKI and chronic kidney diseases was reported in Additional file 1: Table S3, unfortunately, it was not feasible to perform a successful analysis because of the incomplete data.
Sensitivity analysis demonstrated that removing de Abajo et al. [36] (higher mortality rates but fewer patients admitted to ICU in both the intervention and control group) sharply decreased heterogeneity, and the reduction in hospital mortality rate remained statistically significant (RR 0.39; 95% CI 0.25–0.59; P < 0.0001; I2 = 58%) (Additional file 1: Fig. S2). For 30-day mortality, the heterogeneity (I2) decreased from 60% (P = 0.04) to 0 (P = 0.74) without distorting the pooled result (z = 0.59, P = 0.56) when one study [34] was removed. The heterogeneity of IMV use was attributed to the study performed by Abbas Soleimani [33], the origin of heterogeneity might be that the rate of IMV was vastly different in the aforementioned two studies. Adjusted RRs were reported in Additional file 1: Table S4, the differences of 30-day mortality and new or worsening congestive heart failure increased to be statistically significant (both Ps < 0.05).
Meta-regression analysis
Meta-regression analysis was performed to explore the relationship between hospital mortality and the characteristics of the research design and patient baseline characteristics. The RR was found to be significantly affected by the sample size (t = 3.04, P = 0.019) (Additional file 1: Fig. S3A) but not by the publishing year (t = 1.29, P = 0.237) (Additional file 1: Fig. S3B), trial type (t = − 0.30, P = 0.771) (Additional file 1: Fig. S3C), number of research centers (t = 1.14, P = 0.292) (Additional file 1: Fig. S3D), and number of nations (t = − 1.91, P = 0.098) (Additional file 1: Fig. S3E). Multivariate meta-regression indicated these research design characteristics and explained the R2 = 78.78% heterogeneity result among studies on hospital mortality. Univariate meta-regression analysis revealed that the effect of patient baseline characteristics (age, male sex, rate of underlying chronic kidney disease or diabetes) on the association between ACEI/ARB continuation and hospital mortality was not significant (P > 0.05) (Additional file 1: Fig. S4); however, multivariate meta-regression revealed a confident model between hospital mortality and male sex (P = 0.023), and underlying diabetes (P = 0.023).
Publication bias
Based on visual inspection, the funnel plot was slightly asymmetric, thus indicating potential publication bias (Additional file 1: Fig. S5), which was significant when evaluated using Egger’s test (P = 0.042) and not significant when assessed using Begg’s test (P = 0.754).
Discussion
To the best of our knowledge, this is the first meta-analysis study to determine whether ACEIs/ARBs should be continued in patients with previous ACEI/ARB use in both the intervention and control groups before COVID-19 infection. We found that the continuation of ACEIs/ARBs could be maintained as it was associated with lower hospital deaths, ICU admission, and IMV in patients with COVID-19 without significant side effects on other clinical outcomes, although the benefits of continuing previous ACEI/ARB use were mainly shown in observational studies.
The RAAS has become a cause of concern ever since ACE2 was considered the binding receptor of SARS-CoV during the 2003 SARS pandemic [41]. In this COVID-19 pandemic, ACE2 has been implicated again as the key passage of SARS-CoV-2 entry into the host cells but with a 10- to 20-fold higher affinity than SARS-CoV [42]. Additionally, ACE2 has not only been limited to being a receptor; it has also been found to play a crucial role in lung injury caused by both SARS-CoV [43] and SARS-CoV-2 [7]. The RAAS is involved in the process of lung injury through the regulation of the ACE2-Ang-(1–7)-Mas receptor-G protein pathway and/or ACE-Ang II-AT1R pathway [44]. The two homologs interact with each other; ACE2 hydrolyzes Ang II to Ang-(1–7) and whittles the ACE-Ang II-AT1R pathway [45], whereas ACE cleaves Ang I to produce Ang II and counteracts the function of the ACE2-Ang II-Ang-(1–7) access. The imbalance of the two pathways dominates the effect of the RAAS during inflammatory lung injury [46, 47], thus rendering it possible to adopt ACEIs/ARBs or recombinant ACE2 protein as a therapeutic option [48]. Results of animal studies have found that ACEIs potentially reduce Ang-II and increase Ang-(1–7) levels in the plasma and ARBs potentially increase Ang-II, Ang-(1–7) levels, as well as the activity of ACE2. Therefore, ACEIs/ARBs potentially attenuate lung injury by hindering the classical RAAS axis (ACE-Ang II-AT1R) and aggrandizing the new ACE2-Ang II-Ang-(1–7)/Mas receptor pathway [10, 49].
At the clinical level, trials that explored the effect of ACEIs/ARBs on COVID-19-induced lung injury were predominantly performed on patients with hypertension before SARS-CoV-2 infection. Two cohort studies with large sample sizes indicated that taking ACEIs/ARBs before COVID-19 infection could reduce all-cause mortality compared with non-use of ACEIs/ARBs [9, 50]. However, a few meta-analyses found that taking ACEIs/ARBs did not affect mortality significantly [16, 51,52,53,54], but all the included trials compared the effect of ACEIs/ARBs with that of placebos or other anti-hypertension drugs. For these trials, it remains impeachable to draw a conclusion regarding the continuation or discontinuation of previous ACEI/ARB treatment considering the differing ACE2 levels between the two comparison groups. Patients in the control group took placebos or other antihypertensives, and ACE2 expression was not affected by ACEIs/ARBs, whereas ACE2 in intervention group patients was upregulated by ACEIs/ARBs.
The present study further clarified whether ACEIs/ARBs should be continued in patients who possessed the same level of ACE2 in both the intervention and control groups because they all took ACEIs/ARBs before COVID-19 infection. This novelty distinguishes the present study from aforementioned studies. The pooled result of the present study indicated that continued exposure to ACEIs/ARBs was associated with a 41.1% decrease in all-cause hospital mortality, thus corroborating the protective survival effect of continued ACEI/ARB use. This protective effect may be attributed to the multiple inhibition function of ACEIs/ARBs on inflammation induced by the RAAS axis. ACEIs inhibit the conversion of Ang I to Ang II, and ARBs block the attachment of Ang II to AT1R, thus interrupting the ACE-Ang I-Ang II-AT1R inflammatory pathway, with ACEIs/ARBs simultaneously increasing the substrates (Ang I and Ang II) of ACE2. Additionally, ACEIs/ARBs increase ACE2 expression and enhance its activity [55], thus playing a protective role after infection [56]. The increased substrates (Ang I and Ang II) and the enhanced converzyme (ACE2) produce more Ang-(1–7), which strengthens the Ang-(1–7)-Mas receptor anti-inflammatory pathway. Hence, we deemed ACEI/ARB continuation to relieve lung injury and the cytokine storm through correcting the imbalance between the COVID-19-induced inflammatory and anti-inflammatory pathways. This standpoint was verified by the lower rate of ICU admission and lower risk of IMV in the ACEI/ARB continuation group. In patients who took ACEIs/ARBs prior to COVID-19 infection, continued ACEI/ARB use mitigated the severity of COVID-19 and reduced the risk of death. Nonetheless, heterogeneity among studies was high, and further sensitivity analysis found that it predominantly arose from the study by de Abajo et al. [36]. In that study, the definition of (dis)continuation of ACEIs/ARBs was more rigorous, and the patient inclusion criteria were more stringent; additionally, hospital mortality was considerably high in both the ACEI/ARB discontinuation and continuation groups.
Subgroup analysis revealed that the effect of continuing ACE/ARB use in reducing hospital mortality, ICU admission, and IMV was predominantly demonstrated in observational studies; all RCTs found no difference between the continuation and discontinuation use of ACE/ARB. According to the strength of evidence, RCT provides more robust evidence than observational studies. There might be some confounders in the nonrandom trials. The reasons of discontinuing were excavated. Three studies reported that the reasons of ACEI/ARB discontinuation were dependent on clinical need such as hypotension and acute kidney injury [31, 33, 34]. Subgroup analysis was performed on blood pressure and found that the benefits of discontinuation were mainly demonstrated in the studies in which patients in the group who were discontinuing ACEI/ARB had hypotension, which is consistent with clinical medicine. However, it is well known that those who have severe symptoms have a higher risk of bad outcomes due to their serious condition besides the effect of the discontinuation of drugs, which hints that hypotension might be a vital confounding bias that could give misleading results, although baseline blood pressure and kidney function were comparable in all the included RCTs and one observational studies (Additional file 1: Table S2). Nevertheless, the weakness of this evidence cannot be used to give a recommendation to discontinue previous ACEI/ARB treatment as no evidence was found that indicated a disadvantage in continuing ACEI/ARB use [57]. In addition, blind withdrawal of drugs may result in unstable disease control [58, 59]. As a result, ACEI/ARB treatment could be maintained based on the current results of the present study. However, more RCTs should be performed to obtain more robust evidence.
Meta-regression indicated that male sex and diabetes were impact factors, a finding that is inconsistent with that of studies in which the pooled results compared ACIEs/ARBs with placebos or other antihypertensive agents [51]. The negative impact of male sex might have been related to the higher expression of ACE2 [60], which allowed more SARS-CoV-2 to enter the patients’ body and cause more severe disease and higher mortality than that in the female sex [61, 62]. Similarly, ACE2 expression was upregulated approximately 30% in both type 1 [63] and type 2 [64] diabetes. The mechanism underlying the negative effect of diabetes might not have been limited to the higher level of ACE2; a more complicated pathophysiology might have been involved in the disease course of patients COVID-19 and comorbid diabetes [65].
Limitations
Our conclusions were predominantly derived from observational studies, although they were confirmed to be of relatively high quality. More RCTs were required to strengthen the evidence. ACEIs and ARBs elicit different feedbacks in the RAAS, especially the levels of Ang II, Ang-(1–7) ACE, and ACE2 [4], thus potentially making them exert varying effects on inflammation and even the clinical outcomes. However, this study had no means of clarifying this because subgroup analysis based on antihypertensive agents, ACIs, or ARBs could not be performed. The findings might be affected to a certain extent by a confounder (patients had more severe symptoms in the ACEI/ARB discontinuation group). There was no way to elucidate the influence of this confounding factor by multivariate regression analysis with the effect size because limited data could be extracted from the included trials in this meta-analysis study, however, sensitivity analysis by removing a dataset, changing the effect model or introducing adjusted RR as effect size indicated robust and stable findings in most of the outcomes of interest. Additionally, publication bias might have subsisted owing to the divergence between the Egger’s and Begg’s tests.
Conclusions
In patients with COVID-19, previous ACEI/ARB treatment could be continued as the continuation of ACEI/ARB use was associated with reduced hospital mortality, ICU admission, and IMV, without remarkable disadvantage. However, the benefits were mainly shown in observational studies, and simultaneously, the potential confounders should be kept in mind. The effects of the continuation of ACEI/ARB treatment might have also been affected by sample size, male sex, and underlying diabetes mellitus. More multicenter RCTs are warranted to enhance the robustness of evidence.
Availability of data and materials
The dataset supporting the conclusions of this article is available in the personal homepage of the corresponding author, Q.L., at ResearchGate (https://www.researchgate.net/profile/Qi-Liu-169).
Abbreviations
- RAAS:
-
Renin–angiotensin–aldosterone system
- ACE2:
-
Angiotensin-converting enzyme 2
- ACE:
-
Angiotensin-converting enzyme
- Ang I:
-
Angiotensin I
- Ang II:
-
Angiotensin II
- AT1R:
-
Type 1 angiotensin receptor
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- NOS:
-
Newcastle–Ottawa Scale
- COVID-19:
-
Corona virus disease 2019
- ACEIs:
-
Angiotensin-converting enzyme inhibitors
- ARBs:
-
Angiotensin receptor blockers
- CKD:
-
Chronic kidney disease
- ICU:
-
Intensive care unit
- RR:
-
Risk ratio
- CI:
-
Confidence interval
- RCT:
-
Randomized controlled trial
References
Bashir MF, Sadiq M, Talbi B, Shahzad L, Adnan Bashir M. An outlook on the development of renewable energy, policy measures to reshape the current energy mix, and how to achieve sustainable economic growth in the post COVID-19 era. Environ Sci Pollut Res Int. 2022;29:43636–47.
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–93.
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.
Ingraham NE, Barakat AG, Reilkoff R, Bezdicek T, Schacker T, Chipman JG, Tignanelli CJ, Puskarich MA. Understanding the renin–angiotensin–aldosterone-SARS-CoV axis: a comprehensive review. Eur Respir J. 2020;56:1.
Raiden S, Nahmod K, Nahmod V, Semeniuk G, Pereira Y, Alvarez C, Giordano M, Geffner JR. Nonpeptide antagonists of AT1 receptor for angiotensin II delay the onset of acute respiratory distress syndrome. J Pharmacol Exp Ther. 2002;303:45–51.
Gopallawa I, Uhal BD. Molecular and cellular mechanisms of the inhibitory effects of ACE-2/ANG1-7/Mas axis on lung injury. Curr Top Pharmacol. 2014;18:71–80.
South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16:305–7.
Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with covid-19 diagnosis and mortality. JAMA. 2020;324:168–77.
Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–81.
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–10.
Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398-405.
Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3–20.
Karthika T, Joseph J, Das VRA, Nair N, Charulekha P, Roji MD, Raj VS. SARS-CoV-2 cellular entry is independent of the ACE2 cytoplasmic domain signaling. Cells. 2021;10:7.
Onweni CL, Zhang YS, Caulfield T, Hopkins CE, Fairweather L, Freeman WD. ACEI/ARB therapy in COVID-19: the double-edged sword of ACE2 and SARS-CoV-2 viral docking. Crit Care. 2020;24:475.
Wang Y, Chen B, Li Y, Zhang L, Wang Y, Yang S, Xiao X, Qin Q. The use of renin–angiotensin–aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2021;93:1370–7.
Bavishi C, Whelton PK, Mancia G, Corrao G, Messerli FH. Renin–angiotensin-system inhibitors and all-cause mortality in patients with COVID-19: a systematic review and meta-analysis of observational studies. J Hypertens. 2021;39:784–94.
Greco A, Buccheri S, D’Arrigo P, Calderone D, Agnello F, Monte M, Milluzzo RP, Franchina AG, Ingala S, Capodanno D. Outcomes of renin–angiotensin–aldosterone system blockers in patients with COVID-19: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2020;6:335–7.
Grover A, Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2021;7:148–57.
Lee HW, Yoon CH, Jang EJ, Lee CH. Renin–angiotensin system blocker and outcomes of COVID-19: a systematic review and meta-analysis. Thorax. 2021;76:479–86.
Kuster GM, Pfister O, Burkard T, Zhou Q, Twerenbold R, Haaf P, Widmer AF, Osswald S. SARS-CoV2: should inhibitors of the renin–angiotensin system be withdrawn in patients with COVID-19? Eur Heart J. 2020;41:1801–3.
Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA. 2020;323:1769–70.
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–9.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. Newcastle–Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 20 May 2022.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Macedo AVS, de Barros ESPGM, de Paula TC, Moll-Bernardes RJ, MendoncaDosSantos T, Mazza L, Feldman A, Arruda GDS, de Albuquerque DC, de Sousa AS, et al. Discontinuing vs. continuing ACEIs and ARBs in hospitalized patients with COVID-19 according to disease severity: insights from the BRACE CORONA trial. Am Heart J. 2022;249:86–97.
Cannata F, Chiarito M, Reimers B, Azzolini E, Ferrante G, My I, Viggiani G, Panico C, Regazzoli D, Ciccarelli M, et al. Continuation versus discontinuation of ACE inhibitors or angiotensin II receptor blockers in COVID-19: effects on blood pressure control and mortality. Eur Heart J Cardiovasc Pharmacother. 2020;6:412–4.
Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, Mallipattu SK, Skopicki HA, Singer AJ, Duong TQ. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222:1256–64.
Chaudhri I, Koraishy FM, Bolotova O, Yoo J, Marcos LA, Taub E, Sahib H, Bloom M, Ahmad S, Skopicki H, et al. Outcomes associated with the use of renin–angiotensin–aldosterone system blockade in hospitalized patients with SARS-CoV-2 infection. Kidney360. 2020;1:801–9.
Soleimani A, Kazemian S, Karbalai Saleh S, Aminorroaya A, Shajari Z, Hadadi A, Talebpour M, Sadeghian H, Payandemehr P, Sotoodehnia M, et al. Effects of angiotensin receptor blockers (ARBS) on in-hospital outcomes of patients with hypertension and confirmed or clinically suspected COVID-19. Am J Hypertens. 2020;33:1102–11.
Lahens A, Mullaert J, Gressens S, Gault N, Flamant M, Deconinck L, Joly V, Yazdanpanah Y, Lescure FX, Vidal-Petiot E. Association between renin–angiotensin–aldosterone system blockers and outcome in coronavirus disease 2019: analysing in-hospital exposure generates a biased seemingly protective effect of treatment. J Hypertens. 2021;39:367–75.
Aparisi A, Catala P, Amat-Santos IJ, Marcos-Mangas M, Lopez-Otero D, Veras C, Lopez-Pais J, Cabezon-Villalba G, Cacho Antonio CE, Candela J, et al. Chronic use of renin–angiotensin–aldosterone inhibitors in hypertensive COVID-19 patients: results from a Spanish registry and meta-analysis. Med Clin. 2022;158:315–23.
de Abajo FJ, Rodriguez-Miguel A, Rodriguez-Martin S, Lerma V, Garcia-Lledo A, Group M-ACS. Impact of in-hospital discontinuation with angiotensin receptor blockers or converting enzyme inhibitors on mortality of COVID-19 patients: a retrospective cohort study. BMC Med. 2021;19:118.
Sharma A, Elharram M, Afilalo J, Flannery A, Afilalo M, Tselios C, Ni J, Ezekowitz JA, Cheng MP, Ambrosy AP, et al. A randomized controlled trial of renin–angiotensin–aldosterone system inhibitor management in patients admitted in hospital with COVID-19. Am Heart J. 2022;247:76–89.
Bauer A, Schreinlechner M, Sappler N, Dolejsi T, Tilg H, Aulinger BA, Weiss G, Bellmann-Weiler R, Adolf C, Wolf D, et al. Discontinuation versus continuation of renin–angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, andomized, controlled, open-label trial. Lancet Respir Med. 2021;9:863–72.
Cohen JB, Hanff TC, William P, Sweitzer N, Rosado-Santander NR, Medina C, Rodriguez-Mori JE, Renna N, Chang TI, Corrales-Medina V, et al. Continuation versus discontinuation of renin–angiotensin system inhibitors in patients admitted to hospital with COVID-19. A prospective, randomised, open-label trial. Lancet Respir Med. 2021;9:275–84.
Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Dos Santos TM, Mazza L, Feldman A, D’Andrea Saba Arruda G, de Albuquerque DC, Camiletti AS, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–64.
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3.
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–9.
Samavati L, Uhal BD. ACE2, much more than just a receptor for SARS-COV-2. Front Cell Infect Microbiol. 2020;10:317.
Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/angiotensin-(1–7)/MAS axis of the renin–angiotensin system: focus on angiotensin-(1–7). Physiol Rev. 2018;98:505–53.
Gaddam RR, Chambers S, Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy Drug Targets. 2014;13:224–34.
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–6.
Yamaguchi T, Hoshizaki M, Minato T, Nirasawa S, Asaka MN, Niiyama M, Imai M, Uda A, Chan JF, Takahashi S, et al. ACE2-like carboxypeptidase B38-CAP protects from SARS-CoV-2-induced lung injury. Nat Commun. 2021;12:6791.
Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81:537–40.
Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona-Rubio AE, Jacob M, Procop GW, Harrington S, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1020–6.
Singh R, Rathore SS, Khan H, Bhurwal A, Sheraton M, Ghosh P, Anand S, Makadia J, Ayesha F, Mahapure KS, et al. Mortality and severity in COVID-19 patients on ACEIs and ARBs—a systematic review, meta-analysis, and meta-regression analysis. Front Med. 2021;8: 703661.
Dai XC, An ZY, Wang ZY, Wang ZZ, Wang YR. Associations between the use of renin–angiotensin system inhibitors and the risks of severe Covid-19 and mortality in covid-19 patients with hypertension: a meta-analysis of observational studies. Front Cardiovasc Med. 2021;8: 609857.
Baral R, Tsampasian V, Debski M, Moran B, Garg P, Clark A, Vassiliou VS. Association between renin–angiotensin–aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw Open. 2021;4: e213594.
Pranata R, Permana H, Huang I, Lim MA, Soetedjo NNM, Supriyadi R, Soeroto AY, Alkatiri AA, Firman D, Lukito AA. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:983–90.
Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8: e21.
Hanff TC, Harhay MO, Brown TS, Cohen JB, Mohareb AM. Is there an association between Covid-19 mortality and the renin–angiotensin system? A call for epidemiologic investigations. Clin Infect Dis. 2020;71:870–4.
Bidulka P, Fu EL, Leyrat C, Kalogirou F, McAllister KS, Kingdon EJ, Mansfield KE, Iwagami M, Smeeth L, Clase CM, et al. Stopping renin–angiotensin system blockers after acute kidney injury and risk of adverse outcomes: parallel population-based cohort studies in English and Swedish routine care. BMC Med. 2020;18(1):195.
Janse RJ, Fu EL, Clase CM, Tomlinson L, Lindholm B, van Diepen M, Dekker FW, Carrero JJ. Stopping versus continuing renin–angiotensin-system inhibitors after acute kidney injury and adverse clinical outcomes: an observational study from routine care data. Clin Kidney J. 2022;15(6):1109–19.
Xu Y, Fu EL, Trevisan M, Jernberg T, Sjolander A, Clase CM, Carrero JJ. Stopping renin–angiotensin system inhibitors after hyperkalemia and risk of adverse outcomes. Am Heart J. 2022;243:177–86.
Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov. 2020;6:37.
Liu Y, Lu H, Wang W, Liu Q, Zhu C. Clinical risk factors for mortality in patients with cancer and COVID-19: a systematic review and meta-analysis of recent observational studies. Expert Rev Anticancer Ther. 2021;21:107–19.
Mukherjee S, Pahan K. Is COVID-19 gender-sensitive? J Neuroimmune Pharmacol. 2021;16:38–47.
Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop PH, Finniane Study G. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens. 2012;30:375–83.
Rajpal A, Rahimi L, Ismail-Beigi F. Factors leading to high morbidity and mortality of COVID-19 in patients with type 2 diabetes. J Diabetes. 2020;12:895–908.
Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2501800) and Leader Project of Henan Province Health Young and Middle-aged Professor (HNSWJW2020013).
Author information
Authors and Affiliations
Contributions
QL conceived and designed the study, participated in literature search, collected data, performed statistical analysis, interpreted the results, and drafted the manuscript; WF participated in the literature search, collected data, performed statistical analysis, and drafted the manuscript; CZ: helped with literature inclusion, collected data, performed statistical analysis, and drafted the manuscript; ZD and BD helped with literature search, and collected data; BS and RC participated in design and coordination, analyzed the data, interpreted the results, and revised the manuscript; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Subgroup analysis on hospital mortality according to the number of research centers. Figure S2. Sensitivity analysis of heterogeneity among studies on hospital mortality. Figure S3. Meta-regression analysis to find the impact factor of ACEI/ARB continuation on hospital mortality. Figure S4. Univariate meta-regression analysis to find the impact factor of ACEI/ARB continuation on in-hospital mortality. Figure S5. Funnel plots to estimate the publication bias regarding the effect of ACEI/ARB continuation on hospital mortality. Table S1. Reasons of discontinuing ACEi/ARB. Table S2. Blood pressure of the included patients. Table S3. Baseline acute kidney injury and chronic kidney disease. Table S4. The pooled adjusted RR of the outcomes of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Q., Fu, W., Zhu, Cj. et al. Effect of continuing the use of renin–angiotensin system inhibitors on mortality in patients hospitalized for coronavirus disease 2019: a systematic review, meta-analysis, and meta-regression analysis. BMC Infect Dis 23, 53 (2023). https://doi.org/10.1186/s12879-023-07994-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-07994-7