Abstract
Background
Research on the immune response to inactivated COVID-19 vaccination among people living with HIV (PLWH) is limited, especially among those with low CD4+ T lymphocyte (CD4 cell) count. This prospective cohort study aimed to assess the humoral immune response to inactivated COVID-19 vaccination among PLWH compared to HIV negative controls (HNCs) and to determine the impact of CD4 cell count on vaccine response among PLWH.
Methods
The neutralizing antibodies (nAbs) and the specific IgM and IgG-binding antibody responses to the inactivated COVID-19 vaccine at the third month after the second dose of inactivated COVID-19 vaccination were measured among 138 PLWH and 35 HNCs. Multivariable logistic regression and multiple linear regression models were conducted to identify factors associated with the seroconversion rate of antibodies and the magnitude of anti-SARS-CoV-2 antibody titers, respectively.
Results
At the end of the third month after two doses of vaccination, the seroconversion rates of IgG were comparable between PLWH (44.9%; 95% CI 36.5–53.3%) and HNCs (60.0%; 95% CI 42.9–77.1%), respectively. The median titers and seroconversion rate of nAbs among PLWH were 0.57 (IQR: 0.30–1.11) log10 BAU/mL and 29.0% (95% CI 21.3–36.8%), respectively, both lower than those in HNCs (P < 0.05). After adjusting for age, sex, comorbidities, and CD4 cell count, the titers and seroconversion rate of nAbs were comparable between PLWH and HNCs (P > 0.05). Multivariable regression analyses showed that CD4 cell count < 200/μL was independently associated with lower titers and seroconversion rate of nAbs among PLWH (P < 0.05). A positive correlation was observed between the CD4 cell count and nAbs titers in PLWH (Spearman's ρ = 0.25, P = 0.0034).
Conclusion
Our study concluded that the immune response to inactivated COVID-19 vaccination among PLWH was independently associated with CD4 cell count, PLWH with lower CD4 cell count showed a weaker humoral immune response, especially those with CD4 cell count < 200/μL. This finding suggests that expanding COVID-19 vaccination coverage among PLWH is impendency. In addition, aggressive ART should be carried out for PLWH, especially for those with low CD4 cell count, to improve the immune response to vaccines.
Similar content being viewed by others
Introduction
Recently, the ongoing pandemic of coronavirus disease 2019 (COVID-19) has posed a serious threat to global public health and economic development [1]. While it is noteworthy that SARS-CoV-2 infection has brought a big challenge to people living with HIV (PLWH). Studies have reported that PLWH had increased risks of more severe disease and deaths from COVID-19 [2, 3], possibly as a result of immunosuppression, higher rates of multimorbidity, unsuppressed HIV viral load (HIV-VL), and other determinants of health [4,5,6,7].
Nevertheless, limited information is available about the immune response to COVID-19 immunization in PLWH, especially in those with low CD4+ T lymphocyte (CD4 cell) count. Furthermore, the emerging immunogenicity data in PLWH were mostly focused on mRNA COVID-19 vaccines [8,9,10,11] or adenovirus vector-based vaccines [12,13,14], while very few studies focused on the immune response to inactivated COVID-19 vaccines. Two studies that reported the effect of inactivated COVID-19 vaccine on PLWH both had very small sample sizes [15, 16].
WIBP-CorV is an inactivated COVID-19 vaccine. An isolated SARS-CoV-2 strain (WIV‐04) was cultivated in Vero cells, chemically inactivated by β-propiolactone, then mixed with an aluminium-based adjuvant [17, 18]. Phase 1 and 2 trials revealed that WIBP-CorV had a low rate of side effects and good immunogenicity [17]. The interim analysis of phase 3 clinical trials showed that the vaccine is 72.8% effective against the symptomatic COVID-19 cases and 100% against severe disease [19]. WIBP-CorV vaccine was one of the most commonly used vaccine in China.
Previously, another published study of our team based on PLWH and HIV negative controls (HNCs) who finished 70 days’ follow-up showed that early humoral immune response to the inactivated COVID-19 vaccine was weaker and delayed among the PLWH than that among HNCs. But it has not discussed the risk factors associated with the humoral immune response in PLWH and HNCs because of limited sample size [20]. This study aimed to fill this gap by comparing the humoral immune response induced by the inactivated COVID-19 vaccine between PLWH and HNCs, and determining the impact of CD4 cell count on vaccine response in PLWH.
Materials and methods
Study participants and design
The study was conducted from March to October 2021. A total of 138 PLWH and 35 HNCs who received two doses of inactivated COVID-19 vaccine (Sinopharm, WIBP-CorV, 4 µg/0.5 mL, WIV04 strain, Wuhan Institute of Biological Products Co. Ltd) with an interval of 28 days were enrolled in our study. The inclusion criteria for PLWH included the following: (1) age ≥ 18 years old; (2) confirmed HIV infection by HIV-1/2 Western blot assay. Exclusion criteria included the following: (1) presence of severe hearing loss, impaired vision, or intellectual disability observed by the interviewers; or (2) a history of SARS-CoV-2 infection (via serological and nucleic acid test), major psychiatric illness (schizophrenia or bipolar disorder) or neurocognitive impairment; the HNCs shared the first inclusion criteria and both exclusion criteria with PLWH. HNCs were recruited from the physical examination center in Zhongnan Hospital of Wuhan University. Written informed consent was obtained from each participant before screening for eligibility. 138 PLWH and 35 HNCs completed immunizations with inactivated COVID-19 vaccine at respective community hospitals and scheduled visits within the prescribed time. Blood samples were collected at baseline (before the first dose vaccination) and the 3rd month after the second dose of COVID-19 vaccination. Data on demographic information, including age, sex, and comorbidities (i.e., hypertension, diabetes mellitus, hyperlipidemia, cancer, chronic cardiovascular and lung, liver, or kidney diseases) were collected from all participants through an electronic questionnaire before vaccination. Clinical and laboratory data regarding the HIV status of PLWH were obtained from the China National HIV/AIDS Comprehensive Response Information Management System (CRIMS). The CD4 cell count of the PLWH and HNCs were tested with the blood samples at baseline.
Immunogenicity assessments
The primary humoral immunogenicity outcomes included the neutralizing antibodies (nAbs) and the specific IgM and IgG-binding antibody response to the COVID-19 vaccine, measured at baseline and 3rd months after the participants were fully present vacillated with inactivated COVID-19 vaccination. An in-house SARS-CoV-2 nAbs assay kit by surrogate virus neutralization test (Livzon Diagnostics Inc., Zhuhai, China) was used to determine the serum titers of nAbs against the spike protein receptor-binding domain (RBD) according to the manufacturers' instructions. In brief, SARS-CoV-2 surrogate virus neutralization test detects total immunodominant neutralizing antibodies targeting the viral spike (S) protein receptor-binding domain in an isotype- and species-independent manner. This rapid test is based on antibody-mediated blockade of the interaction between the angiotensin-converting enzyme 2 (ACE2) receptor protein and the receptor-binding domain [21], a positive response is defined as ≥ 10BAU/mL. The semi-quantitative of total specific IgM and IgG antibodies were detected using an in-house-developed ELISA kits (Livzon Diagnostics Inc.,Zhuhai, China), which used the recombinant nucleocapsid (N) and RBD antigen of SARS-CoV-2 as coating antigen, following the instruction manual. Positive responses of IgM and IgG were defined as ≥ 0.15 EU/mL and 0.18 EU/mL, respectively. The qualitative of specific IgM or IgG antibodies was detected using an in-house-developed colloidal gold kit (Livzon Diagnostics Inc.,Zhuhai, China), following the instruction manual. We defined seroconversion of antibodies as a change from baseline seronegative to seropositive.
Statistical analysis
Categorical variables were presented as n (%) and compared using the Chi-square test or Fisher's exact test. Continuous variables with normal distribution were presented as mean (standard deviation [SD]) and compared using t-test or ANOVA analysis, while continuous variables with abnormal distribution were expressed as median (interquartile range [IQR]) and compared using Mann–Whitney U test. Multivariable logistic regression models with 2-sided 95% confidence intervals were conducted to identify factors associated with the seroconversion rate of antibodies. Multiple linear regression was employed to identify factors associated with the magnitude of anti-SARS-CoV-2 antibody titers. Analyses were conducted using SPSS software, version 26.0 (IBM SPSS Inc), and GraphPad Prism 8 for Mac OS X (GraphPad Software, San Diego, CA, USA). A two-sided p < 0.05 was considered statistically significant.
Results
Study participants
Characteristics of the 138 PLWH and 35 HNCs were shown in Table 1. PLWH and HNCs were similar in age and comorbidities but differed in proportion of male (P < 0.001). The median (IQR) age of PLWH was 38 (31–49) years old, and 88.4% were males. 91.3% of the PLWH were receiving ART and 107 (77.5%) had a HIV VL < 50 copies /mL. The CD4 cell count in PLWH was significantly lower than that in HNCs [495(IQR: 320–646) vs. 666 (IQR: 534–800)/μL, P < 0.001].
Binding-antibody responses to COVID-19 vaccination
At the end of third month after two doses of vaccination, the seroconversion rates of IgM in PLWH and HNCs were 3.6% (95% CI 0.5–6.8%) and 2.9% (95% CI 0–8.7%), respectively, while no significant difference between the two groups was observed. No significant difference was also found in seroconversion rates of IgG between PLWH (44.9%; 95% CI 36.5–53.3%) and HNCs (60.0%; 95% CI 42.9–77.1%). After adjusting for age, sex, comorbidities, and CD4 cell count, IgG seroconversion rates were comparable between PLWH and HNCs.
For the difference of IgG titers between PLWH and HNCs, univariate analysis showed that the IgG titers among PLWH was significantly lower than that among HNCs, but no significantly difference was found after adjusting for age, sex, comorbidities, and CD4 cell count.
Neutralizing antibody responses to COVID-19 vaccination among PLWH and HNCs
At the end of third month after two doses of vaccination, the seroconversion rate of nAbs among PLWH was 29.0% (95% CI 21.3–36.7%), which was significantly lower than that among HNCs (48.6%; 95% CI 31.2–66.0%)]. The nAbs titers among PLWH [0.57 (IQR: 0.30–1.11) log10 BAU/mL] was also significantly lower than that among HNCs [(median 0.91; IQR, 0.64–1.26) log10 BAU/mL] (Fig. 1).
nAbs titers in different groups (PLWH were divided into three groups: CD4 cell count < 200/μL, CD4 cell count between 200 and 500/μL and CD4 cell count ≥ 500/μL). P < 0.05, nAbs titers in PLWH were significantly different from HNCs; P < 0.01, nAbs titers in PLWH with CD4 cell count < 200/μL were differ from those in PLWH with CD4 cell count ≥ 500/μL; P > 0.05, there were no significantly difference between the groups; P-values were computed using the Mann–Whitney U-test
In multivariable logistic regression analysis, the people with CD4 cell count < 200/μL tended to have a lower seroconversion rate of nAbs (OR: 0.09; 95% CI 0.01–0.74; P = 0.03), as compared to those with CD4 cell count ≥ 500 /μL. Age, sex, comorbidities and HIV infection were not significantly associated with the seroconversion rate of nAbs (Table 2).
We further transformed the nAbs titers (log10) and performed multivariable linear regression analysis. The results determined that nAbs titers in participants with CD4 cell count < 200/μL were − 0.21 log10 lower than those with CD4 cell count ≥ 500/μL (P = 0.012). Age, sex, comorbidities, and HIV infection were not significantly associated with the nAbs titers (Table 3).
Then we analyzed the association between IgG and nAbs, the result showed that there were positive correlations between IgG and nABs titers in PLWH (ρ = 0.843, p < 0.001) and HNCs (ρ = 0.766, p < 0.001).
Neutralizing antibody responses to COVID-19 vaccination among PLWH
At the end of third month after two doses of vaccination, the seroconversion rates of nAbs were 5.6% (95% CI 0–17.3%) in the group with CD4 cell count < 200/μL, 25.0% (95% CI 12.8–37.2%) in the group with CD4 cell count between 200 and 500/μL, and 61.8% (95% CI 49.9–73.6%) in the group with CD4 cell count ≥ 500/μL, respectively. In the multivariable model, participants with CD4 cell count < 200/μL tend to have a lower nAbs seroconversion rate than those with CD4 cell count ≥ 500/μL (P = 0.03) (Table 4).
At the end of third month after two doses of vaccination, the median nAbs titers were 0.30 (IQR: 0.30–0.59) log10 BAU/mL in the group with CD4 cell count < 200/μL, 0.61 (IQR: 0.30–1.14) log10 BAU/mL in the group with CD4 cell count between 200 and 500/μL and 0.81 (IQR: 0.35–1.24) log10 BAU/mL in the group with CD4 cell count ≥ 500/μL, respectively. The nAbs titers were significantly different in three CD4 groups (P = 0.009), while participants with lower CD4 cell count < 200 /μL tend to have lower nAbs titers (Fig. 1). Multivariable linear regression analysis confirmed this finding (Table 5). There was no significant association between age, sex, comorbidities, HIV-VL, ART, and nAbs titers (P > 0.05).
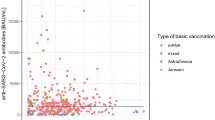
The correlation analysis between CD4 cell count and nAbs titers showed a positive correlation in PLWH (Spearman's ρ = 0.25, P = 0.0034), while no significant correlation between CD4 count and nAbs titers was observed in HNCs (Spearman's ρ = 0.03, P = 0.86) (Fig. 2).
Discussion
Understanding the humoral immune response induced by the inactivated COVID-19 vaccine and the impact of CD4 cell count on vaccine response in PLWH were essential in decision-making regarding future disease control and revaccination strategies. It is important to ensure adequate protection against infection in the vulnerable population, especially to prevent the emerging new variants. This prospective cohort study extends the existing literatures [8,9,10,11,12,13,14,15,16] by providing more comprehensive evidence to assess the inactivated COVID-19 vaccine response among PLWH.
We found that PLWH and HNCs had a similar humoral immune response to the inactivated COVID-19 vaccine at the 3rd month after two doses of inactivated COVID-19 vaccination. Even though nAbs titers and seroconversion rate of nAbs in PLWH were both lower than that in HNCs, after adjusting for potential confounders, the differences disappeared. These findings are consistent with the results of other studies conducted in South Africa and UK, which suggested that the immune responses produced by the adenovirus vector-based COVID-19 vaccine among PLWH are similar to those among HNCs [12, 13]. Other studies about the immune response to mRNA COVID-19 vaccine among PLWH also reported similar humoral immune response to the healthy controls [8]. The results indicate that PLWH should complete both doses of inactivated COVID-19 vaccine to achieve good protection. Studies have shown that two doses of inactivated CoronaVac vaccines offer high levels of protection against severe disease and death among all age group [22].
Several studies have shown that PLWH have lower responses to some types of vaccine, including hepatitis A, hepatitis B, and influenza vaccine. These responses are dependent on the level of CD4 cell count [23,24,25]. CD4 cell is pivotal in orchestrating both the humoral and cellular immune responses to vaccination and has an essential impact on antibody production [26]. Some studies also suggested that PLWH with low CD4 cell count had a poor response to the COVID-19 vaccine while PLWH with CD4 cell count in a healthy range mounted equivalent vaccine responses to those in HIV-negative people [27, 28]. Our study found a statistically lower titer and seroconversion rate of nAbs among PLWH with the CD4 cell count < 200 μL (versus the group CD4 ≥ 500/μL). We also found a positive correlation between CD4 cell count and nAbs titers in PLWH and CD4 cell count < 200/μL independently predicted lower nAbs titers. The results indicate that PLWH, especially those with CD4 cell count < 200/μL were still relatively vulnerable even after two doses of inactivated COVID-19 vaccination. A study on the infection forms of SARS-CoV-2 infection among PLWH showed that PLWH were more likely to be an asymptomatic carrier [29]. Prolonged SARS-CoV-2 infection in advanced PLWH with profound immunosuppression or without ART would drive SARS-CoV-2 virus evolution [30], which may be the reason that 'omicron' emerged. We should expand COVID-19 vaccination coverage and promote the uptake among the lower- and middle-income countries where the COVID-19 vaccination rates are still low [31], and especially among PLWH. Furthermore, we should strengthen the appropriate ART for PLWH, especially for those with low CD4 cell count, to increase the CD4 cell count and strengthen their immune response level to vaccines and achieve longer duration of vaccines. This is not just to prevent PLWH from SARS-CoV-2 infection but to prevent the emergence of new variants.
This study has several limitations. First, the sample size of HNCs was relatively small. Studies with larger sample size will be more conductive to identify individuals who are particularly vulnerable to the impact of SARS-CoV-2 infection and develop targeted vaccination interventions. Second, imbalance existed in the sex distribution of PLWH, which may lead to some bias in our results. However, a previous study found the responses to inactivated COVID-19 vaccination had no significant differences between male and female, which may mitigate some of the sex imbalance in this study [19]. Third, the T-cell responses against the inactivated COVID-19 vaccines weren’t investigated in our study. Long-term follow-up for PLWH with inactivated COVID-19 vaccination will be performed in our further study, and the durability and quality of humoral and cellular responses of inactivated COVID-19 vaccines will be evaluated.
In conclusion, our study indicated that PLWH with lower CD4 cell count showed a weaker humoral immune response to inactivated COVID-19 vaccination, especially those with CD4 cell count < 200 /μL. Additional measures against COVID-19 are needed for PLWH who have low CD4 cell count.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PLWH:
-
People living with HIV
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- HNCs:
-
HIV negative controls
- nAbs:
-
The neutralizing antibodies
- CRIMS:
-
China National HIV/AIDS Comprehensive Response Information Management System
- RBD:
-
Spike protein receptor-binding domain
- ACE2:
-
Angiotensin-converting enzyme 2
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- NNRTIs:
-
Nonnucleoside reverse transcriptase inhibitors
- INSTIs:
-
Integrase inhibitors
- PIs:
-
Protein inhibitors
- NVP:
-
Nevirapine
- EFV:
-
Efavirenz
- EVG:
-
Elvitegravir
- DTG:
-
Dolutegravir
- LPV/r:
-
Lopinavir/ritonavir
References
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–36. https://doi.org/10.1001/jama.2020.6019.
Guo W, Ming F, Dong Y, Zhang Q, Liu L, Gao M, et al. Driving force of Covid-19 among people living with HIV/AIDS in Wuhan. China AIDS Care. 2022;23:1–8. https://doi.org/10.1080/09540121.2022.2052259.
Yang X, Sun J, Patel RC, Zhang J, Guo S, Zheng Q, et al. Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV. 2021;8(11):e690–700. https://doi.org/10.1016/s2352-3018(21)00239-3.
Tesoriero JM, Swain CE, Pierce JL, Zamboni L, Wu M, Holtgrave DR, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open. 2021;4(2):e2037069. https://doi.org/10.1001/jamanetworkopen.2020.37069.
Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) clinical characterization protocol (UK): a prospective observational study. Clin Infect Dis. 2021;73(7):e2095–106. https://doi.org/10.1093/cid/ciaa1605.
Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8(1):e24–32. https://doi.org/10.1016/S2352-3018(20)30305-2.
Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2021;73(7):e2005–15. https://doi.org/10.1093/cid/ciaa1198.
Levy I, Wieder-Finesod A, Litchevsky V, Biber A, Indenbaum V, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect. 2021;27(12):1851–5. https://doi.org/10.1016/j.cmi.2021.07.031.
Woldemeskel BA, Karaba AH, Garliss CC, Beck EJ, Wang KH, Laeyendecker O, et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with human immunodeficiency virus (HIV). Clin Infect Dis. 2022;74(7):1268–70. https://doi.org/10.1093/cid/ciab648.
Ruddy JA, Boyarsky BJ, Bailey JR, Karaba AH, Garonzik-Wang JM, Segev DL, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS. 2021;35(14):2399–401. https://doi.org/10.1097/QAD.0000000000003017.
Ruddy JA, Boyarsky BJ, Werbel WA, Bailey JR, Karaba AH, Garonzik-Wang JM, et al. Safety and antibody response to the first dose of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine in persons with HIV. AIDS. 2021;35(11):1872–4. https://doi.org/10.1097/qad.0000000000002945.
Madhi SA, Koen AL, Izu A, Fairlie L, Cutland CL, Baillie V, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV. 2021;8(9):e568–80. https://doi.org/10.1016/S2352-3018(21)00157-0.
Frater J, Ewer KJ, Ogbe A, Pace M, Adele S, Adland E, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8(8):e474–85. https://doi.org/10.1016/S2352-3018(21)00103-X.
Khan K, Lustig G, Bernstein M, Archary D, Cele S, Karim F, et al. Immunogenicity of SARS-CoV-2 infection and Ad26.CoV2.S vaccination in people living with HIV. Clin Infect Dis. 2021. https://doi.org/10.1093/cid/ciab1008.
Lv Z, Li Q, Feng Z, Zheng X, Nayin, Yang H, et al. Inactivated SARS-CoV-2 vaccines elicit immunogenicity and T-cell responses in people living with HIV. Int Immunopharmacol. 2022;102:108383. https://doi.org/10.1016/j.intimp.2021.108383.
Feng Y, Zhang Y, He Z, Huang H, Tian X, Wang G, et al. Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: a non-randomized cohort study. EClinicalMedicine. 2022;43:101226. https://doi.org/10.1016/j.eclinm.2021.101226.
Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–60.
Wang ZJ, Zhang HJ, Lu J, Xu KW, Peng C, Guo J, et al. Low toxicity and high immunogenicity of an inactivated vaccine candidate against COVID-19 in different animal models. Emerg Microbes Infect. 2020;9:2606–18.
Al Kaabi KN, Zhang Y, Xia S, Yang Y, Al QM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45.
Zou S, Wu M, Ming F, Wu S, Guo W, Marley G, et al. Immune response and safety to inactivated COVID-19 vaccine: a comparison between people living with HIV and HIV-naive individuals. AIDS Res Ther. 2022;19(1):33. https://doi.org/10.1186/s12981-022-00459-y.
Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, Hu Z, Chen VC, Young BE, Sia WR, Tan YJ, Foo R, Yi Y, Lye DC, Anderson DE, Wang LF. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–8. https://doi.org/10.1038/s41587-020-0631-z.
Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, Sans C, Leighton P, Suárez P, García-Escorza H, Araos R. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–84. https://doi.org/10.1056/NEJMoa2107715.
Fritzsche C, Bergmann L, Loebermann M, Glass A, Reisinger EC. Immune response to hepatitis A vaccine in patients with HIV. Vaccine. 2019;37(16):2278–83. https://doi.org/10.1016/j.vaccine.2019.02.064.
Leggat DJ, Iyer AS, Ohtola JA, Kommoori S, Duggan JM, Georgescu CA, et al. Response to pneumococcal polysaccharide vaccination in newly diagnosed HIV-positive individuals. J AIDS Clin Res. 2015. https://doi.org/10.4172/2155-6113.1000419.
Seremba E, Ocama P, Ssekitoleko R, Mayanja-Kizza H, Adams SV, Orem J, Katabira E, Reynolds SJ, Nabatanzi R, Casper C, Phipps W. Immune response to the hepatitis B vaccine among HIV-infected adults in Uganda. Vaccine. 2021;39(8):1265–71. https://doi.org/10.1016/j.vaccine.2021.01.043.
Brenna E, McMichael AJ. The importance of cellular immune response to HIV: implications for antibody production and vaccine design. DNA Cell Biol. 2022;41(1):38–42. https://doi.org/10.1089/dna.2021.0520.
Brumme ZL, Mwimanzi F, Lapointe HR, Cheung P, Sang Y, Duncan MC, et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines. 2022;7(1):28. https://doi.org/10.1038/s41541-022-00452-6.
Antinori A CS, Meschi S. Immunogenicity of mRNA vaccination against SARS-CoV-2 in persons living with HIV (PLWHs) with low CD4 count or previous AIDS. 18th European AIDS Conference, EACS.2021, London Abstract OS4/3.
Wu M, Ming F, Wu S, Liu Y, Zhang X, Guo W, et al. Risk of SARS-CoV-2 infection among people living with HIV in Wuhan, China. Front Public Health. 2022;10:833783. https://doi.org/10.3389/fpubh.2022.833783.
Cele S, Karim F, Lustig G, San JE, Hermanus T, Tegally H, et al. SARS-CoV-2 prolonged infection during advanced HIV disease evolves extensive immune escape. Cell Host Microbe. 2022;30(2):154–62. https://doi.org/10.1016/j.chom.2022.01.005.
The Washington Post. As omicron variant is detected around the world, travel bans may be too late, experts say. https://www.washingtonpost.com/world/2021/11/28/omicron-variant-coronavirus/. Accessed 29 Nov 2021. 2021.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Nature Science Foundation of China (81903371), NIMH (R34MH119963), the National Science and Technology Major Project (2018ZX10101-001-001-003), Special Found on Prevention and Control of New Coronary Pneumonia in Guangdong Universities (2020KZDZX1047), Medical Science and Technology Innovation Platform Support Project of Zhongnan Hospital, Wuhan University (PTXM2020008), Science and Technology Innovation Cultivation Fund of Zhongnan Hospital, Wuhan University (cxpy2017043), Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (TFJC2018004) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2020-PT320-004).
Author information
Authors and Affiliations
Contributions
KL and WT participated in the inception of the idea of this manuscript with lead roles in conducting the study; SW, SZ and FM involved in the data analysis and drafting of the manuscript; SW, SZ and FM involved in data collection and interpretation; MW, WG, JL, ZX and ZZ participated in discussion section. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Research and Ethics Committee of Zhongnan Hospital (2020079K-1). All the procedures were performed in accordance with the Declaration of Helsinki, and the patient's confidentiality was maintained throughout the investigation. All individuals provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, S., Zou, S., Ming, F. et al. Humoral immune response to inactivated COVID-19 vaccination at the 3rd month among people living with HIV. BMC Infect Dis 23, 34 (2023). https://doi.org/10.1186/s12879-023-07982-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-07982-x