Abstract
Background
Despite the development and application of vaccines against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) around the world, the scientific community is still trying to find some therapies to avoid or ameliorate the fatal evolution of the Coronavirus disease 2019 (COVID-19). Since the publication of the potential use of ivermectin as a treatment against the disease, a pleiad of information about it has been published. However, the evidence is not strong or weak enough to conclude its usefulness in the clinical evolution of patients infected with SARS-CoV-2. We evaluate the efficacy and safety of ivermectin in the treatment of Mexican patients with asymptomatic and mild COVID-19 in a three-day administration in comparison to placebo.
Methods
A randomized, double-blind, placebo-controlled trial was carried out in 66 adults with asymptomatic and mild COVID-19. Patients were randomly assigned 1:1 ratio to ivermectin plus acetaminophen or placebo plus acetaminophen. The primary endpoint was the proportion of subjects without a disease progression to severity according to COVID-19 guidelines by the National Institutes of Health (NIH) since randomization to 14 days.
Results
None of the participants presented progression to a severe state in either group. Viral load was measured on Days 1, 5, and 14. No significant differences were observed in baseline or 14-day between groups (p = 0.720 and 0.362, respectively). However, on Day 5, a significant difference in viral load was observed between groups (p = 0.039). The frequency of symptoms was similar between groups, and no significant differences were observed. The most frequent symptom was cough. One severe adverse event associated with SARS-CoV-2 infection was observed in the ivermectin group.
Conclusions
At standard doses, ivermectin is not effective to prevent progression to a severe state or reducing symptoms in adults with asymptomatic and mild COVID-19.
Trial registration The study was registered with ClinicalTrial.gov (NCT04407507) on May 29, 2020.
Similar content being viewed by others
Background
Despite the development and application of vaccines against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) around the world, the scientific community is still trying to find therapies to avoid or ameliorate the fatal evolution of Coronavirus disease 2019 (COVID-19). Since the publication of the potential use of ivermectin as a treatment against the disease [1], much information about it has been published. However, the evidence is not strong or weak enough to conclude its usefulness in the clinical evolution of patients infected with SARS-CoV-2 [2]. Ivermectin has been described as a broad-spectrum antiviral, inhibiting nuclear import due to its ability to inactivate host nuclear transport proteins, such as integrase and NS5, limiting the ability of West Nile virus to infect at low concentrations [5]. It also inhibits the replication of yellow fever virus and other viruses, such as dengue, likely by attacking nonstructural helicase 3 activity [4].
The aim of the study was to evaluate the efficacy and safety of ivermectin in the treatment of patients with asymptomatic and mild COVID-19 in a three-day administration in comparison to placebo.
Methods
Study design and participants
The protocol was approved by the local ethics, biosafety, and investigation committees of the Investigación Biomédica para el Desarrollo de Fármacos S.A de C.V. and the Mexican health ministry Federal Commission for Protection against Sanitary Risks(COFEPRIS): 203301410A0055. The procedures were conducted in compliance with the Helsinki Declaration and Good Clinical Practice guidelines. Written informed consent was obtained from all patients. The study is registered on ClinicalTrials.gov: NCT04407507. The study adheres to Consolidated Standards of Reporting Trials (CONSORT) guidelines and includes a completed CONSORT checklist as an Additional file 1.
A randomized, double-blind, placebo-controlled trial was conducted to determine the efficacy and safety of ivermectin among subjects with asymptomatic and mild COVID-19. Subjects were included in two different sites in Guadalajara and Zapopan, Mexico: Hospital Hispano and Investigacion Biomedica para el Desarrollo de Farmacos (Ibiomed).
Participants
Eligible participants were > 18-year-old men and women diagnosed with SARS-CoV-2 infection by real-time polymerase chain reaction (RT–PCR) testing of nasopharyngeal swab samples. We considered viral load undetectable when the threshold cycle (Ct) value of the nucleocapsid (N) gene from SARS-CoV-2 was ≥ 40. Patients with moderate or severe COVID-19 [9], diagnosis of other respiratory infections, impaired liver function tests (> 5 times above the normal level of alanine aminotransferase or aspartate aminotransferase), history of recurrent urinary tract infections, pregnancy or nursing women, active participation in other clinical trials, and use of antibiotics, verapamil, cyclosporine A, trifluoperazine or antiparasitic treatment for a concomitant disease were excluded. Moreover, subjects with a reported allergy or sensitivity to ivermectin, or acetaminophen, or its use during the protocol were also excluded.
Randomization
Participants were randomly allocated 1:1 to receive oral ivermectin or placebo using a macro in Microsoft Excel (version 16.38; Microsoft Corporation, Redmond, WA, USA) with random numbers. The investigators or study coordinators enrolled and assigned participants to intervention. An unblinded pharmacist provided masked intervention according to permuted blocks of 2 in the randomization sequence. The rest of the clinical staff, investigators, and participants were blinded to the assignment.
Interventions
Patients received 12 mg per day of ivermectin tablets or placebo for 3 days. Both groups received 500 mg acetaminophen tablets four times a day for 14 days to eliminate symptom bias. Ivermectin, Acetaminophen (Pharmacen, Laboratories Alpharma), and the placebo were provided by Ibiomed.
Outcomes measures
The primary endpoint was the proportion of subjects without disease progression to severity according to COVID-19 guidelines by the NIH [9] from randomization to 14 days. Severity was considered if the participants had an oxygen saturation (SpO2) < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg, a respiratory rate > 30 breaths/min, or lung infiltrates > 50%.
Secondary endpoints were the indirect analysis of the viral load using the threshold cycle (Ct) value of the nucleocapsid (N) gene from SARS-CoV-2 which is inversely related to the viral load, and the presence and frequency of COVID-19 symptoms were measured on Days 1, 5 and 14. Subjects were asked to complete a diary of symptoms and adverse events for 14 days. They recorded the presence of the following symptoms: fever, cough, muscular pain, fatigue, shortness of breath, headache, diarrhea, palpitations, expectoration, and “other”. In the “others” field question, several subjects answered hypogeusia/ageusia, hyposmia/anosmia, and back pain.
Vital signs (temperature, blood pressure, pulse rate, oxygen saturation, and respiratory rate) and RT–PCR were measured on Days 1, 5, and 14. Laboratory tests were performed at baseline and Day 14. A security follow-up phone call was performed on Day 21.
Sample size and statistical analysis
The sample size was calculated according to the study by Wölfel et al. [10] We considered the viral RNA concentrations isolated in the throat and nasopharyngeal samples at the beginning of the infection and the difference of 10,000 copies at 10 days. A sample size of 54 patients provided 80% power to detect a 0.10 absolute difference in the proportions of the placebo group using a 2-sided test with a significance level of 0.05. The sample size was inflated to a total of 66 participants to allow for 20% dropouts.
An exploratory analysis was carried out to identify the nature of the variables and their distribution. All tests were 2-tailed. For the quantitative variables, Kolmogorov–Smirnov tests were used to identify whether they adjusted to the normality assumptions with a 95% confidence interval. The statistical analysis to compare the difference of means between groups was calculated with a t-test, and the ordinal variables were analyzed with the Chi2 test. Values of p ≤ 0.05 were considered significant. We used IBM SPSS software (version 26, IBM Corporation, Armonk, NY, USA) for all statistical analyses.
Results
Patients’ characteristics
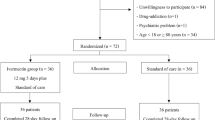
From 2020 July 21 to 2021 January 9, 104 subjects were screened, and 66 subjects were enrolled to receive either ivermectin (n = 33) or placebo (n = 33). Of these subjects, 10 were excluded from the efficacy analysis because nine of them were enrolled without a positive SARS-CoV-2 test, and one withdrew consent after the first visit (Fig. 1). However, they were included in the safety analysis.
The demographic and baseline characteristics of the group are listed in Table 1. The comparative analysis of the baseline measurements showed no differences between study groups.
Primary endpoint
None of the participants presented progression to a severe state in either group from baseline to Day 14.
Viral load
The cycle threshold (Ct) value for gene N was measured on Days 1, 5, and 14, and no significant differences were observed between the placebo and ivermectin groups at baseline (23.3 ± 5.15 vs. 26.2 ± 6.36; p = 0.720) or 14 days (32.94 ± 7.74 vs. 33.74 ± 4.77; p = 0.362). However, on Day 5, a significant difference was observed between groups (28.25 ± 4.21 vs. 30.64 ± 3.74; p = 0.039) (Fig. 2A). On Day 5, viral load was undetectable in 13.3% of patients in the ivermectin arm and in 7.7% of patients in the placebo arm. On Day 14, the ivermectin-treated group reached a 28% negative rate, while 23% of the subjects treated with placebo presented negative results; no significant differences were observed between the two arms (p = 0.560). Both the ivermectin and placebo groups exhibited significant differences in the proportion of negative subjects between Days 1 and 14 (23% in placebo and 28% in ivermectin) (Fig. 2B).
Analysis of the results of the SARS-CoV-2 diagnostic test by RT–PCR in subjects who received ivermectin and placebo on Days one, five and 14. A The Ct values are presented as the means (standard deviation) analyzed with Student’s t-test. B Diagnosis of the RT–PCR in percentage of subjects analyzed with * Pearson χ2 test. *, p < 0.05
Symptoms
Compliance to the diary was 96% and 92.9% in the placebo and ivermectin groups, respectively (X2 = 2.23; p = 0.525). The data are presented as the percentage of days answered by each group.
Cough, fatigue, myalgia, and headache were the most frequent symptoms. Regarding asymptomatic and symptomatic subjects (Fig. 3), on day one, 13.8% of the subjects who received ivermectin were asymptomatic, and 4% received placebo. At day five, asymptomatic patients accounted for 12% and 18% on placebo and ivermectin, respectively. On Day 14, these values reached 45.8% in those who received placebo and 37% in those who received ivermectin, and no significant difference was observed between groups regarding this distribution.
Symptom frequency in subjects who received ivermectin and placebo. Each bar corresponds to the percentage of subjects who reported the symptoms by day in placebo or ivermectin (white bars; asymptomatic; black bars 1–3 symptoms, grey bars 4–6 symptoms, and light grey ≥ 7 symptoms reported by day). From left to right are the values of days one, five and 14, respectively. X axis; percentage of subjects, Y axis; days and treatment. Pearson χ2 test
We also classified symptoms according to the number of symptoms per subject (1 to 3, 4 to 5, and ≥ 7 symptoms reported per day), showing the expected progressive decrease in the number of symptoms reported per day over time for both arms.
Regarding vital signs, the participants in the ivermectin group presented a higher heart rate on day 5 (72.78 ± 15.7 vs 83.46 ± 10.5 bpm; p = 0.007), but without a significant difference in comparison to baseline (p = 0.873) (Additional file 2: Table S1).
Safety
Overall, 30 adverse events were reported, 17 in the placebo group and 13 in those treated with ivermectin. The majority of events were associated with COVID-19, but no differences were observed in the proportion of events that occurred between groups (Table 2). In the ivermectin group, a serious adverse event occurred; encephalitis secondary to SARS-CoV-2 occurred on study Day 10, and the subject made a full recovery. Regarding clinical laboratory tests, some differences were observed between baseline and Day 14 posttreatment: cholesterol and platelets increased in both the placebo and ivermectin groups. An increment in erythrocytes was observed from 5.05 ± 0.52 M/µL to 6.84 ± 2.60 M/µL (p = 0.002) from baseline to Day 14 (Additional file 2: Table S2).
Discussion
In this clinical trial of patients with asymptomatic and mild COVID-19, those who were randomized to receive ivermectin for 3 days in the early stages of diagnosis at a dose of 12 mg/day presented a reduction on viral load within the five days after starting treatment in relation to the group that received placebo.
These results agree with those observed by Ahmed et al. [11], who evaluated the effect of ivermectin alone and in combination with doxycycline compared to placebo in hospitalized COVID-19 patients. They observed that treatment with ivermectin for 5 days resulted in earlier viral clearance (9.7 days) than the group treated in combination (11.5 days) or with placebo (12.7 days). A significant difference was observed against placebo at 7 and 14 days. In concordance, Pott-Junior et al. [12] found shorter times for obtaining two consecutive negative SARS-CoV-2 RT–PCR tests in subjects treated with ivermectin compared to those who received standard of care at hospital admission.
Chaccour et al. [13] published a pilot study of 24 patients, where they observed no difference in viral clearance after treatment with ivermectin at a single dose of 400 mcg/day or placebo in patients with mild symptoms. They reported that 7 days after treatment, all subjects remained with a positive SARS-CoV-2 test in the N gene; however, in both the N and E genes, a decrease in viral load was observed in those treated with ivermectin on days 4 and 7 post-treatment compared to placebo. These observations were accompanied by lower IgG antibody titters in the ivermectin group on Day 21 post-treatment. Similar results were reported in Lebanon by Samaha et al. [14] in a pilot clinical trial. They described a significant difference in Ct values between patients who received ivermectin compared to placebo from Day 0 to Day 3.
This effect could be associated in a directly proportional way to the dose, since a preliminary report indicated that providing ivermectin two times the dose that we administered (24 mg/day) for a single day translated into a higher proportion of subjects negative for COVID-19 at day five compared to 12 mg/day or placebo. In this trial, no statistical significance was observed; thus, it could be assumed that an indication of ivermectin for more days is required to reach such significance [15]. However, our study did not show a correlation between the calculated dose (12 mg between weight in kilograms) and the Ct value of the N gene (data not shown). According to Schmith et al. [16] report, single repeated doses of 200 mcg/kg, 120 mg weekly, or 60 mg every 72 h were not enough to reach the concentrations relative to the 50% inhibition (IC50) established by Caly et al. [3], but they did not intend consecutive daily doses as we did. The diverse results with similar or different posology could be explained considering the pharmacokinetics (PK) of ivermectin [17]. Absorption is intestinal, and diarrhea is a common clinical manifestation of COVID-19. This finding decreases the absorption rate and bioavailability and therefore the effect. Moreover, the elimination is principally in feces. However, there is no report of individual results of the effect or presence of diarrhea, and our data did not show any differences in Ct value between those who presented diarrhea and those who did not on any of the evaluated days (data not shown).
Similarly, a randomized study in hospitalized patients showed a benefit in the Ct value of patients treated with ivermectin for 7 days (100, 200, or 400 mcg/kg) in relation to those who did not receive ivermectin, with no-dose behavior.
Our findings and the others previously mentioned can potentially be translated into avoiding progression to severe disease. According to Liu's and Samaha's report, there is a relationship between the Ct value and the intensity of the disease [14]. Furthermore, other studies have shown that receiving ivermectin as part of COVID-19 treatment is associated with a lower mortality rate, accompanied by lower levels of inflammatory biomarkers, such as C-reactive protein, ferritin, and D-dimer [18].
In this study, an evaluation of the presence of symptoms associated with COVID-19 was carried out through the delivery of a diary; in general, the participants had an adherence to the completion of the symptom diary greater than 80%. The analysis revealed that the subjects who received ivermectin presented a higher frequency of symptoms from basal evaluation, of which fatigue and diarrhea were reported as adverse events expected from receiving ivermectin in the Food and Drug Administration (FDA) technical data sheet. In addition, in the same document, muscle pain and headache have also been observed in clinical trials [21]. Therefore, we could assume that these may be due to the drug and not the disease.
These findings do not correspond to the results by Chaccour et al. [13] in his pilot study, since they observed a lower frequency of the following symptoms in those treated with ivermectin: cough, anosmia, hyposmia, and shortness of breath. They did not observe any differences in the frequency of fever or an increase in the frequency of gastrointestinal symptoms by 3.5 times [11]. Recently, a randomized clinical trial by Shahbaznejad et al. [22] reported that patients who used ivermectin at a single dose, calculated according to weight, decreased the hospital stay and duration of symptoms compared to the control group.
On the other hand, a randomized trial with 400 patients, in which the time to resolution of symptoms was evaluated in subjects who received ivermectin (300 mcg/kg) for 5 days, did not observe a significant difference between groups, with a time to resolution slightly lower in the placebo arm (10 vs. 12 days) [23].
The evidence available to date seems to indicate that there is little or no benefit in the resolution of symptoms associated with the consumption of ivermectin at a standard dose [24].
Regarding safety issues, no relationship was found between the drug and any adverse event. The encephalitis event that occurred in the subject who received ivermectin was diagnosed as secondary to SARS-CoV-2 infection, a phenomenon that has been previously documented [25].
Taken together, these data do not show that ivermectin is effective in the treatment of COVID-19 by "accelerating" viral clearance in the first week, which may translate into a lower rate of complications. However, clinical trials with a larger number of participants are required at different doses and times of administration to elucidate the treatment with greater efficacy and a better safety profile. A limitation of the present study was the small sample size, especially for the interpretation of safety outcomes.
Conclusions
At standard doses, ivermectin is not effective to prevent progression to a severe state or reducing symptoms in adults with mild COVID-19.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 19
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus-2
- NIH:
-
National Institutes of Health
- COFEPRIS:
-
Federal Commission for Protection against Sanitary Risks
- CONSORT:
-
Consolidated Standards of Reporting Trials
- RT-PCR:
-
Reverse Transcription-Polymerase Chain Reaction
- SpO2 :
-
Peripheral oxygen saturation
- PaO2/FiO2 :
-
Persistent arterial partial pressure of oxygen/fraction of inspired oxygen
- Ct:
-
Threshold cycle
- RNA:
-
Ribonucleic acid
- bpm:
-
Beats per minute
- IC50 :
-
Inhibitory concentration
- PK:
-
Pharmacokinetics
- FDA:
-
Food and Drug Administration
References
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
World Health Organization. Coronavirus disease (COVID-2019) situation report. 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-july-2021.
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787.
Hariyanto TI, Halim DA, Rosalind J, Gunawan C, Kurniawan A. Ivermectin and outcomes from Covid-19 pneumonia: a systematic review and meta-analysis of randomized clinical trial studies. Rev Med Virol. 2021. https://doi.org/10.1002/rmv.2265.
Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med. 2020;17:e1003501.
Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot. 2020;73:593–602.
Yang SNY, Atkinson SC, Wang C, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177: 104760.
Crump A. Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J Antibiot. 2017;70:495–505.
National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) treatment guidelines. 2019. https://www.covid19treatmentguidelines.nih.gov/.
Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–9.
Ahmed S, Karim MM, Ross AG, et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–6.
Pott-Junior H, Bastos Paoliello MM, Miguel AQC, et al. Use of ivermectin in the treatment of Covid-19: a pilot trial. Toxicol Rep. 2021;8:505–10.
Chaccour C, Casellas A, Blanco-Di Matteo A, et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. EClinicalMedicine. 2021;32: 100720.
Samaha AA, Mouawia H, Fawaz M, et al. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in lebanon. Viruses. 2021;13:989.
Mohan A, Tiwari P, Suri T, et al. Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial. Prepr Res Sq. 2021. https://doi.org/10.21203/rs.3.rs-191648/v1.
Schmith VD, Zhou JJ, Lohmer LRL. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin Pharmacol Ther. 2020;108:762–5.
Canga AG, Prieto AMS, Liébana MJD, Martínez NF, Vega MS, Vieitez JJG. The pharmacokinetics and interactions of ivermectin in humans–a mini-review. AAPS J. 2008;10:42–6.
Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–7.
Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter J-J. ICON (Ivermectin in COvid Nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19. medRxiv. 2020. https://doi.org/10.1101/2020.06.06.20124461.
Okumuş N, Demirtürk N, Çetinkaya RA, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21:411.
Merck & Co. Stromectrol. FDA approved package insert 2009. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050742s026lbl.pdf.
Shahbaznejad L, Davoudi A, Eslami G, et al. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin Ther. 2021. https://doi.org/10.1016/j.clinthera.2021.04.007.
López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325:1426–35.
Popp M, Stegemann M, Metzendorf M-I, Gould S, Kranke P, Meybohm P, Skoetz N, Weibel S. Ivermectin for preventing and treating COVID-19. Cochrane Database System Rev. 2021;7:CD015017.
Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–6.
Garg RK, Paliwal VK, Gupta A. Encephalopathy in patients with COVID-19: a review. J Med Virol. 2020;93:206–22.
Acknowledgements
We thank the participants included in the study. We also thank Ana Alcaráz Ledón for her advice on regulatory affairs.
Funding
Investigación Biomédica para el Desarrollo de Fármacos S.A. de C.V. covered all the expenses generated during the study.
This trial was supported Investigación Biomédica para el Desarrollo de Fármacos S.A. de C.V. The funding source participated in study design, data collection, data analysis, data interpretation, or writing of the report, since most of the team are affiliated, but no commercial purposes are intended with this trial results.
Author information
Authors and Affiliations
Contributions
dlRC, MSAR and CLM: Conceptualization, methodology, formal analysis, investigation, writing original draft visualization, and validation. VLB and GMSC: writing review and editing. HPCV, MJ, SOA, PRAM, LVRA, MAAI, VIB, IPC, and RGD: resources, formal analysis, software and methodology. TVA, LGN, MVM GDGA, VRCG, MCJ and RBJG: writing review, conceptualization, funding acquisition and supervision. All authors contributed to the interpretation of the results, provided critical revisions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was approved by the local ethics, biosafety and investigation committees and the Mexican health ministry COFEPRIS: 203301410A0055. The study is registered on ClinicalTrials.gov: NCT04407507. Patients gave their written consent prior the participation on the study and informed consent was obtained from all the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
CONSORT checklist.
Additional file 2.
Supplementary tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de la Rocha, C., Cid-López, M.A., Venegas-López, B.I. et al. Ivermectin compared with placebo in the clinical course in Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial. BMC Infect Dis 22, 917 (2022). https://doi.org/10.1186/s12879-022-07890-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07890-6