Abstract
Background
The relationship between in-utero antiretroviral (ARV) drug exposure and child growth needs further study as current data provide mixed messages. We compared postnatal growth in the first 18-months of life between children who are HIV-exposed uninfected (CHEU) with fetal exposure to ARV drugs (prophylaxis or triple-drug therapy (ART)) and CHEU not exposed to ARVs. We also examined other independent predictors of postnatal growth.
Methods
We analysed data from a national prospective cohort study of 2526 CHEU enrolled at 6-weeks and followed up 3-monthly till 18-months postpartum, between October 2012 and September 2014. Infant anthropometry was measured, and weight-for-age (WAZ) and length-for-age (LAZ) Z-scores calculated. Generalized estimation equation models were used to compare Z-scores between groups.
Results
Among 2526 CHEU, 617 (24.4%) were exposed to ART since -pregnancy (pre-conception ART), 782 (31.0%) to ART commencing post-conception, 879 (34.8%) to maternal ARV prophylaxis (Azidothymidine (AZT)), and 248 (9.8%) had no ARV exposure. In unadjusted analyses, preterm birth rates were higher among CHEU with no ARV exposure than in other groups. Adjusting for infant age, the mean WAZ profile was lower among CHEU exposed to pre-conception ART [-0.13 (95% confidence interval − 0.26; − 0.01)] than the referent AZT prophylaxis group; no differences in mean WAZ profiles were observed for the post-conception ART (− 0.05 (− 0.16; 0.07)), None (− 0.05 (− 0.26; 0.16)) and newly-infected (− 0.18 (− 0.48; 0.13)) groups. Mean LAZ profiles were similar across all groups. In multivariable analyses, mean WAZ and LAZ profiles for the ARV exposure groups were completely aligned. Several non-ARV factors including child, maternal, and socio-demographic factors independently predicted mean WAZ. These include child male (0.45 (0.35; 0.56)) versus female, higher maternal education grade 7–12 (0.28 (0.14; 0.42) and 12 + (0.36 (0.06; 0.66)) versus ≤ grade7, employment (0.16 (0.04; 0.28) versus unemployment, and household food security (0.17 (0.03; 0.31). Similar predictors were observed for mean LAZ.
Conclusion
Findings provide evidence for initiating all pregnant women living with HIV on ART as fetal exposure had no demonstrable adverse effects on postnatal growth. Several non-HIV-related maternal, child and socio-demographic factors were independently associated with growth, highlighting the need for multi-sectoral interventions. Longer-term monitoring of CHEU children is recommended.
Similar content being viewed by others
Background
Maternal triple antiretroviral therapy (ART) scale-up for prevention of vertical HIV transmission has improved maternal health and reduced new pediatric infections to < 1% in high income countries [1] and to < 5% in most low and middle income countries with breastfeeding populations [2, 3]. Therefore, most children affected by the HIV pandemic are now HIV-exposed uninfected (CHEU). Furthermore, strengthening ART programs will increase the likelihood that women living with HIV (WLHIV) will conceive subsequent pregnancies on ART. Taken together, the population of children who are HIV- and ART-exposed during critical intrauterine, intrapartum, and postpartum periods will continue to increase as more children are ART-exposed from conception.
There is ongoing discussion about maternal ART effects on birth and postnatal outcomes, with some studies reporting increased adverse birth outcomes, including preterm birth, low birthweight and small for gestational age [4,5,6,7,8], and poorer postnatal growth with in-utero ART exposure [9,10,11]. Given that the current global goal is to move beyond survival of CHEU and ensure that they have optimal health and wellbeing, more data are required on short and long-term health outcomes of these children. Data from South Africa are particularly important as it is home to a quarter of CHEU globally (estimated at 3.8 million in 2019) [12]. The country also has the largest ART programme [13], and antenatal HIV prevalence has remained around 30% for the past decade, though incident pediatric HIV infections have declined [14]. Furthermore, relationships between HIV and ART exposure and postnatal growth need further exploration in a context of endemic undernutrition and overnutrition [15, 16], childhood illnesses such as diarrhoea [17], syndemic interactions [18], including non-HIV related risk factors such as poverty and inequality [19].
We compared postnatal growth in the first 18-months of life between CHEU with fetal exposure to antiretroviral (ARV) drugs as prophylaxis or as triple-drug combinations intended as therapy (ART) and CHEU without fetal exposure to ARVs. We also examined independent associations of other maternal, child, household, and socio-demographic factors with postnatal growth.
Methods
Study design, setting and methods
The 2012 South African Prevention of Mother to Child Transmission of HIV Evaluation (SA-PMTCT-E) was a nationally-representative health facility-based cross-sectional study that enrolled 6-week (range 4–8 weeks) old infants attending immunization clinics in 9 South African provinces, with the primary aim to measure national PMTCT programme’s early effectiveness [20, 21]. In brief, nurse data collectors sampled mother-infant pairs systematically in large facilities (where participants were recruited at selected fixed intervals based on the target sample) or consecutively in small facilities (where all eligible participants were recruited until target sample obtained) during 6-week immunization visits. Sick infants needing emergency care or hospitalization were excluded.
Infant HIV exposure was used as a marker of maternal HIV status. This was established through antibody testing [Genscreen HIV1/2 Ab EIA (enzyme immunoassay) Bio-Rad and confirmatory Vironostika HIV Uni-form II plus O, bioMérieux, France] on infant dried blood spots. Infant HIV infection status was assessed using polymerase chain reaction (PCR) testing [COBAS AmpliPrep/COBAS TaqMan assay, Roche, New Jersey] on the same dried blood spots [2].
Infants whose mothers reported living with HIV or infants with a positive 6-week HIV antibody test regardless of maternal self-reported HIV status, were eligible for recruitment into a prospective cohort study, nested within a national cross-sectional survey, to measure vertical transmission risk until 18-months postpartum. Recruitment was from 29 October 2012 to 31 May 2013, with follow-up until September 2014. Detailed cohort methods are described elsewhere [2]. In brief, consenting mother-infant pairs were followed at 3, 6, 9, 12, 15 and 18-months during scheduled facility visits coinciding with routine childcare appointments. Infant blood specimens collected between 6-weeks and 15-months underwent afore-mentioned diagnostic tests. At the 18-month visit, study nurses documented results from routinely-administered HIV-1 rapid test (SD Bioline HIV 1/2 3.0 Titma Health, Pty) on the child.
Exposure measures and covariates
Our primary exposure of interest was fetal exposure to maternal ARV, based on self-reported maternal ARV drug use data obtained using a structured questionnaire. During the study period, the national PMTCT programme was implementing CD4 count criteria-based life-long maternal ART initiation (CD4 cell counts ≤ 350 cells/mm3) and infant prophylaxis (World Health Organization (WHO) PMTCT policy Option A: 1 April 2010- 31 March 2013). Women with CD4 cell counts > 350 cells/mm3 were given Azidothymidine (AZT) prophylaxis from 14 weeks gestation. In April 2013, the PMTCT programme transitioned to lifelong ART for all pregnant and lactating WLHIV (WHO PMTCT Option B+) [22, 23]. ART regimens generally consisted of Nevirapine, Tenofovir, and Lamivudine or Emtricitabine [24]. We classified reported maternal ARV exposure as follows in analyses: (1) “pre-conception ART” when ART was started before pregnancy, (2) “post-conception ART” when ART was started during pregnancy, (3) “AZT only” when only AZT-based prophylaxis was given, (4) “None” when a known WLHIV took no ARV drugs during pregnancy, and (5) “Newly-infected” when women reported to have been HIV-negative during pregnancy but their children’s 6-week HIV antibody test result was positive.
Study questionnaires also included questions on self-reported 24-h and 1-week child feeding practices, maternal (Tuberculosis (TB), HIV, CD4 count, syphilis) and child (coughing, diarrhea) morbidity and treatment, maternal obstetric history, socio-demographics characteristics, and peripartum community social support at each time point.
Outcomes
Trained nurse data collectors collected anthropometric data using standardised procedures based on WHO guidelines [25]. Child weight was measured using calibrated A&D personal precision weight scales (UC-321) and length using SECA portable baby length boards (SCA417BLM). Measurements were recorded in kilograms or centimeters to two decimal places. Birthweight, birth length, and gestational age were extracted from participant-held health booklets.
We defined low birthweight (LBW) as birthweight < 2.5 kg; preterm birth (PTB) as birth before 37 completed weeks gestation; and small for gestational age (SGA) as birthweight for gestational age Z-score below − 1.28 [26]. We estimated birthweight and length–for-gestational-age Z-scores using Intergrowth international standards for assessing newborn size for term and pre-term infants [27] and LMSgrowth [28], and excluded gestational ages outside of range for these standards (20 to 24 weeks). We estimated weight-for-age (WAZ), weight-for-length (WLZ), and length-for-age (LAZ) Z-scores at each postnatal timepoint using WHO growth standards [29]. We considered infants as underweight and stunted if their WAZ and LAZ were below -2 standard deviations respectively [25].
Anthropometric measurements and Z-scores were flagged based on criteria (Additional file 1: Box 1), and set to missing if no plausible explanation was established.
Statistical analysis
We performed statistical analyses using STATA standard edition version 15. We calculated frequencies for categorical variables and means (standard deviations) or medians (inter quartile range) for continuous variables. Proportions were compared using Pearson chi-squared test while F-test was used for comparing means. Generalized estimation equations, with a gaussian distribution, were used for univariable and multivariable regression analyses to account for correlations between repeated anthropometric measurements within the same participant. Model covariates were selected based on literature (Additional file 1: Fig. S1) [30] and quasi-likelihood under an independence model criterion. These criteria were also used to inform the best fitting final multivariable models, and to select the independent correlation structure [31]. Mean WAZ and LAZ over the study period were modelled on exposure group, infant age (in months) and important covariates including maternal age, education, employment status, syphilis and TB status, mode and place of delivery, birth attendant, household food security, housing type (brick or non-brick), access to flush toilet and electricity, infant breastfeeding, sex and geographic location. These covariates were treated as potential confounders in the adjusted models assessing the association between foetal ARV exposure and postnatal growth. They were also included as predictors in the multivariable predictive models assessing factors independently associated with study outcomes. Presence of effect measure modification was also explored. We excluded variables that were affected by exposures of interest and shared common causes with outcomes (i.e., LBW, SGA and PTB) from models to minimize bias introduced by adjustment of potential mediators in the presence of unmeasured common causes [32, 33]. We did not include survey sampling weights in final analyses because this adjustment (1) did not change the findings and (2) generally increases standard errors. Instead, we added province in models to adjust for the survey structure. Point estimates were calculated with 95% confidence intervals. Analyses only included CHEU and children were censored if they tested HIV PCR positive at last HIV negative PCR result or died (censored at time point when death was reported). Although statistical testing was performed at the 5% statistical significance level, results were interpreted primarily based on the precision of the estimates.
Results
Participant characteristics
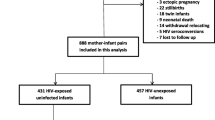
A total of 9120 children were recruited at the 6-week visit, of which 2877 were classified as CHEU children. 2526 CHEU were retained for analysis after excluding 67 CHEU with positive PCR results at 6-weeks or earlier, 25 CHEU with rejected 6-week PCR results, 13 CHEU with equivocal 6-week PCR results, 77 CHEU with missing 6-week PCR results, 70 CHEU with no consent for follow-up, 81 CHEU with missing ARV exposure data, and 18 CHEU with no in-utero HIV and ARV exposure data (Additional file 1: Fig. S2). Thirty CHEU who tested PCR positive postnatally were censored. Among the CHEU, 617 (24.4%) were born to WLHIV who initiated ART pre-conception, 782 (31.0%) were born to WLHIV who initiated ART post-conception, 879 (34.8%) were exposed only to AZT as prophylaxis, 189 (7.5%) were born to WLHIV who did not receive any ARV drugs, and 59 (2.3%) were born to women who reported being HIV-negative and had no ARV drugs during pregnancy, but whose children tested antibody positive at 6 weeks. ARV exposure status was treated as a constant as few women moved across exposure groups during follow-up (Additional file 1: Table S3), making possibility of misclassification bias minimal.
Baseline socio-demographic characteristics of exposure groups are shown in Table 1. WLHIV who initiated ART pre-conception were generally older, multiparous, and employed. They had more ANC visits, caesarian sections (c-section), and hospital-based deliveries, and reported higher rates of TB or syphilis than other exposure groups. WLHIV who initiated ART post-conception had the lowest median (interquartile rage) CD4 cell count [271 (186–340)] followed by those who initiated ART post-conception [350 (250–500)]. Mothers of children exposed to AZT had higher education levels, more births attended by a nurse and a higher median CD4 count than women in ART groups. Newly-infected mothers were younger, primiparous, most likely to have household access to electricity and least likely to report TB or syphilis during pregnancy. Peripartum social support, infant Nevirapine prophylaxis, household piped water or food insecurity, and the child’s sex did not differ between groups.
Adverse birth outcomes
Mean birthweight, birthweight-for-gestational age Z-score and birth length did not differ between ARV exposure groups (Table 2). However, the proportion of PTB was higher among children born to newly-infected women than in other groups.
Mean WAZ (6 weeks to 18 months)
In unadjusted analyses, CHEU exposed to ART pre-conception tended to have the lowest mean WAZ profile while CHEU exposed to in-utero AZT tended to have the highest mean WAZ profile (Fig. 1). In an analysis only adjusting for child age, the mean (95% confidence interval) WAZ profile for children born to WLHIV who initiated ART pre-conception [− 0.13 (− 0.26; − 0.01)] was lower than for CHEU exposed to AZT (Table 3). Although this association between ART and mean WAZ remained after adjusting for several covariates, it was attenuated in the final multivariable model including provincial location (Table 3). Mean WAZ profiles for CHEU with foetal exposure to ART post-conception and those exposed to AZT were parallel. These profiles were also similar between the ARV exposed and unexposed groups (Table 3 and Fig. 1). Several socio-economic, child and maternal factors were independently associated with an increase in mean WAZ in the predictive multivariable model, including having a male child, older maternal age, higher maternal education, maternal employment, c-section delivery, birth attendance by a nurse, household electricity, and household food security. Maternal TB infection, home-based delivery, and breastfeeding were associated with lower mean WAZ. Mean WAZ also differed by geographical location with provinces such as Free State, Gauteng, Northern Cape, North West and Western Cape showing a lower mean WAZ than Eastern Cape (Table 4). Analyses stratified by province showed mean differences in WAZ between ARV groups in some provinces (Free State, Mpumalanga, and North West) but not in others (Gauteng, Eastern Cape, KwaZulu-Natal, Limpopo, Northern Cape and Western Cape), yielded inconsistent associations between ARV exposure and mean WAZ (data not shown). The proportion of underweight children did not differ between exposure groups (Additional file 1: Table S1).
Mean weight-for-age and length-for-age Z-scores from 6-weeks to 18-months by antiretroviral exposure groups, 2012–2014, South Africa. ANC: antenatal care; ART: antiretroviral therapy (Maternal ART regimens generally consisted of Tenofovir, Lamivudine or Emtricitabine and Nevirapine); AZT: Azidothymidine; HEU: HIV exposed uninfected; None: children with no foetal ARV exposure
Mean LAZ (6 weeks to 18 months)
The mean LAZ profiles of the exposure groups overlapped (in time and means) in the unadjusted and adjusted analyses (Fig. 1, Table 3). Several factors were independently associated with a higher mean LAZ in the multivariable model, including having a male child, higher maternal education and maternal employment (Table 5). In contrast home-based delivery, breastfeeding and household food insecurity were associated with lower mean LAZ. Furthermore, CHEU living in provinces except Western Cape had lower mean LAZ than CHEU in Eastern Cape. Although analyses stratified by province showed mean differences in LAZ between ARV groups in some provinces (Eastern Cape, Free State, Gauteng, Limpopo and Mpumalanga), these differences were inconsistent across provinces. There was therefore no clear evidence of an association between ARV exposure and mean LAZ in analyses stratified by province. Evidence showed consistent associations between mean LAZ and socio-demographic and health factors such as maternal education and employment status, place of delivery, food security, breastfeeding, child sex and maternal TB status within provinces (data not shown). The proportion of stunted children also did not differ between exposure groups (Additional file 1: Table S2).
Discussion
Short and long-term health outcomes of CHEU require close monitoring, particularly in settings with high antenatal HIV prevalence and ART coverage such as South Africa. In this national prospective cohort study of CHEU, we found no evidence supporting a detrimental effect of fetal exposure to maternal ARV on birthweight, birthweight-for-gestational age Z-score and birth length. The PTB proportion tended to be higher among children born to women who had received no ART, particularly those who were thought to be newly-infected. Women who had not received ART also reported fewer ANC visits and more frequent home deliveries. The lower antenatal attendance and unmanaged HIV could have contributed to the higher PTB among this group. These findings emphasize the importance of closing HIV testing and ART initiation gaps in healthcare services.
In unadjusted analysis, the mean WAZ profile was lower among children with fetal exposure to maternal ART from pre-conception than in children with foetal exposure to maternal AZT prophylaxis started in pregnancy. We hypothesize that the direction of this association may be explained by confounding by severity of disease and may not be a true effect of ART exposure. PMTCT policy during the study period recommended ART initiation only for women with CD4 cell counts ≤ 350 cells/mm3 and AZT for those with higher CD4 cell counts [24]. As expected women who initiated ART pre-conception had the lowest median CD4 cell count and highest rates of TB and syphilis in our study population. The small mean WAZ profile difference between the pre-conception ARV and AZT exposure groups was attenuated in the models adjusting for confounders. Our data showed no association between mean WAZ and timing of ART initiation. The mean LAZ profile also did not vary between exposure groups. These findings are consistent with data from other South African studies showing that birthweight [34, 35] and postnatal growth [35, 36] outcomes did not vary with fetal exposure to maternal ART, but contrast with data from Ethiopia [37]. Taken together these data provide supporting evidence for initiating pregnant WLHIV immediately on ART, in line with current PMTCT policy guidelines [38].
Our data highlight other important non-HIV specific factors that are associated with growth of CHEU [39]. Mean WAZ was positively associated with some maternal (older age, higher education, and employment) and household (electricity and food security) factors. These findings support global evidence that, in addition, to nutrition-specific solutions [40], indirect non-health-care sector interventions, such as household food security, are crucial for improving child health outcomes [41]. Mean WAZ was also positively associated with male sex. Given that Z-scores are standardized for biological sex, sex may be a proxy for socio-behavioral factors, such as childcare practices, in our study sample. Our data show that maternal TB co-infection was associated with lower mean WAZ. Pregnant WLHIV have a higher likelihood of being co-infected with TB, which is associated with adverse birth outcomes such as LBW [42]. Prevention and management of other infectious diseases is therefore crucial, particularly in pregnant WLHIV. Optimal breastfeeding in the first few months of life is also important for child growth and development [43]. Our finding that breastfeeding is associated with lower mean WAZ has been previously observed among South African children [44]. This observation is likely due to earlier solid food introduction by women who do not breastfeed, which is associated with higher weight velocity and related overnutrition in children [44]. Mean WAZ also differed by geographical location with provinces such as Free State, Gauteng, Northern Cape, North West and Western Cape showing a lower mean WAZ than Eastern Cape. These unexpected observations may partly be explained by geospatial variation in the sampling participants within the provinces. In some provinces participants were sample from rural facilities while in others they were recruited from urban clinics. Findings may also reflect differences in access to healthcare and social protection services, poverty levels, hunger etc. within the provinces which would affect provincial estimates. Our observations corroborate data from nationally-representative studies [45,46,47]. Similar associations were observed for mean LAZ with some slight differences.
Our study has several strengths. First, this is the first study reporting on 18-month postnatal growth patterns of CHEU from a national sample in South Africa. This enabled us to assess the relationship between growth and individual-level factors and to describe growth across geographical locations. Second, study data were collected before universal ART roll-out, enabling us to compare growth of children by fetal exposure exposed to ART, AZT prophylaxis and unmanaged HIV. This comparison is important as the PMTCT programme still has HIV testing and treatment gaps that drive pediatric HIV infections and other adverse outcomes [2, 48]. Third, we used robust lab-confirmed HIV antibody and virological tests to ascertain infant HIV exposure and infection status respectively [49, 50]. Fourth, availability of rich socio-demographic, maternal and infant data enabled us to explore effects of these non-HIV related factors on CHEU growth in addition to in-utero ARV exposure. Fifth, these data, from a national cohort in a middle-income setting, add to the sparse data from large studies in these settings.
Our study has some limitations. First, as adverse perinatal outcome risk may vary by ART drug combinations [51], lack of much variability in the ART regimens used over this period precluded our ability to assess relationships between exposure to specific ARV drugs and child growth. Hence, while our study provides data regarding the effects of Nevirapine-based ART, they may not be generelisable to current Efavirenz- or Dolutegravir-based ART regimens. Second, as maternal ARV use was self-reported, there is a small chance of non-differential misclassification, albeit unlikely as women on AZT in our study had higher median CD4 cell counts than women on ART. Third, we could not adequately adjust for CD4 cell count because these data were missing for 37% participants, reflecting gaps in routine service delivery. We also had missing data for participants who missed all postnatal study visits. Fourth, viral load data were not collected. We also did not collect information on the assessment method used to ascertain gestational age. Given that most public health facilities in South Africa use the last menstrual period method, with ultrasound reserved for complicated pregnancies, we speculate that our data was subject to some measurement bias. Fifth, although feeding data were collected at all timepoints, we used 6-weeks feeding in models because of high rates of mixed feeding from 3 months. Sixth, there is likely bias by indication in our growth comparison by our ARV exposure variable, as ART initiation was CD4 cell count based. Seventh, the primary study was designed to generate national estimates, limiting inter- and intra-provincial growth comparisons. Eighth, the study had no control group of children who are HIV-unexposed, precluding comparisons against background growth patterns. Ninth, there is possible selection bias as the primary study excluded sick infants and those that died before 6 weeks of age. These infants could have been more likely to have adverse birth (e.g. preterm birth) and postnatal (e.g. stunting) outcomes. Tenth, as the definition for newly-infected mothers was partly based on self-reported HIV maternal status during pregnancy, there is possibility some participants in this exposure group may have been misclassified. We believe the probability of misclassification is small as most of these women still thought they were still HIV negative at the 4–8-weeks postpartum. Therefore, most of them had not gone for HIV-related healthcare by that timepoint.
Conclusions
In conclusion, our national cohort study showed postnatal growth of CHEU did not vary by fetal exposure to maternal ARVs. Together with data from other studies [34,35,36], these findings provide supporting evidence for current recommendations about the importance of ensuring that all pregnant WLHIV are on ART. Given that the goal is to ensure that children thrive and reach their potential, longer-term monitoring for potential neurodevelopmental or other health outcomes among CHEU is required. Such monitoring should include non-HIV related maternal, child and socio-demographic risk factors for poorer child growth, which were associated with growth faltering in our data. These factors can only be mitigated through multi-sectoral collaboration and by implementing nutrition-sensitive and -specific interventions tailored for local contexts.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- Ab:
-
Antibody
- ANC:
-
Antenatal care
- ARV:
-
Antiretroviral
- ART:
-
Antiretroviral treatment
- AZT:
-
Azidothymidine
- EIA:
-
Enzyme immunoassay
- GEE:
-
Generalized estimation equation
- HEU:
-
HIV-exposed uninfected
- HIV:
-
Human immunodeficiency virus
- LAZ:
-
Length-for-age Z-score
- LBW:
-
Low birth weight
- PCR:
-
Polymerase chain reaction
- PMTCT:
-
Prevention of Mother to Child Transmission of HIV
- PTB:
-
Preterm birth
- SAPMCTE:
-
South African Prevention of Mother to Child Transmission of HIV Evaluation
- SD:
-
Standard deviation
- SGA:
-
Small for gestational age
- TB:
-
Tuberculosis
- WAZ:
-
Weight-for-age Z-score
- WHO:
-
World health organization
- WLHIV:
-
Women living with HIV
References
Peters H, Francis K, Sconza R, Horn A, Peckham SC, Tookey PA, et al. UK mother-to-child HIV transmission rates continue to decline: 2012–2014. Clin Infect Dis. 2017;64(4):527–8.
Goga AE, Lombard C, Jackson D, Ramokolo V, Ngandu NK, Sherman G, et al. Impact of breastfeeding, maternal antiretroviral treatment and health service factors on 18-month vertical transmission of HIV and HIV-free survival: results from a nationally representative HIV-exposed infant cohort, South Africa. J Epidemiol Community Health. 2020;74(12):1069–77.
World Health Organization, United Nations Children’s Fund. Guideline updates on HIV and infant feeding feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva, Switzerland: World Health Organization; 2016.
Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206(11):1695–705.
Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375(18):1726–37.
Martin F, Taylor GP. Increased rates of preterm delivery are associated with the initiation of highly active antiretrovial therapy during pregnancy: a single-center cohort study. J Infect Dis. 2007;196(4):558–61.
Powis KM, Kitch D, Ogwu A, Hughes MD, Lockman S, Leidner J, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis. 2011;204(4):506–14.
Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS. 2004;18(17):2337–9.
le Roux SM, Abrams EJ, Donald KA, Brittain K, Phillips TK, Nguyen KK, et al. Growth trajectories of breastfed HIV-exposed uninfected and HIV-unexposed children under conditions of universal maternal antiretroviral therapy: a prospective study. Lancet Child Adolesc Health. 2019;3(4):234–44.
Powis KM, Smeaton L, Hughes MD, Tumbare EA, Souda S, Jao J, et al. In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. AIDS. 2016;30(2):211–20.
Powis KM, Smeaton L, Ogwu A, Lockman S, Dryden-Peterson S, van Widenfelt E, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr. 2011;56(2):131–8.
UNAIDS. Number of HIV-exposed children who are uninfected 2021 [Available from: https://aidsinfo.unaids.org/.
UNAIDS. People living with HIV receiving ART (#) 2021. https://aidsinfo.unaids.org/.
Woldesenbet SA, Kufa T, Lombard C, Manda S, Ayalew K, Cheyip M, et al. The 2017 National Antenatal Sentinel HIV Survey, South Africa. Pretoria: National Department of Health; 2019.
National Department of Health, Statistics South Africa, South African Medical Research Council, ICF. South Africa Demographic and Health Survey 2016: Key Indicator Report. Pretoria, South Africa; Rockville, Maryland, USA; 2019.
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81.
National Department of Health. 3rd triennial report of the committee on morbidity and mortality in children under 5 year (COMMIC):2017–2017 Pretoria: National Department of Health; 2017.
Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389(10072):941–50.
Sulla V, Zikhali P. Overcoming poverty and inequality in South Africa: an assessment of drivers constraints and opportunities. Washington: World Bank Group; 2018.
Goga AE, Dinh TH, Jackson DJ, Lombard C, Delaney KP, Puren A, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child, South Africa. J Epidemiol Community Health. 2015;69(3):240–8.
Goga AE, Dinh TH, Jackson DJ, Lombard CJ, Puren A, Sherman G, et al. Population-level effectiveness of PMTCT Option A on early mother-to-child (MTCT) transmission of HIV in South Africa: implications for eliminating MTCT. J Glob Health. 2016;6(2): 020405.
National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV(PMTCT) and the management of HIV in children, adolescents and adults. Pretoria: National Department of Health; 2014 24 December 2014.
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013.
National Department of Health, South African National AIDS Council. Clinical guidelines: PMTCT (Prevention of Mother-to- Child Transmission) In: National Department of Health, editor. Pretoria2010.
World Health Organization. WHO Child Growth Standards: Training Course on Child Growth Assessment. Geneva; 2008.
World Health Organization. Guidelines on optimal feeding of low birthweight infants in low-and middle-income countries. Geneva: World Health Organization; 2011.
Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–68.
Pan H, Cole TJ. LMSgrowth, a Microsoft Excel add-in to access growth references based on the LMS method. Version 2.77 2012. http://www.healthforallchildren.co.uk/.
World Health Organization. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva, Switzerland: World Health Organization; 2006.
Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737.
Cui J. QIC program and model selection in GEE analyses. Stata J. 2007;7(2):209–20.
Hernandez-Diaz S, Schisterman EF, Hernan MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164(11):1115–20.
Wilcox AJ. On the importance–and the unimportance–of birthweight. Int J Epidemiol. 2001;30(6):1233–41.
Malaba T, Phillips T, Le Roux S, Petro G, Brittain K, Zerbe A, et al., editors. Timing of ART initiation in pregnancy and birth outcomes in South African Women. Western Cape Department of Health Research Day 2016; Cape Town.
Nyemba DC, Kalk E, Madlala HP, Malaba TR, Slogrove AL, Davies MA, et al. Lower birth weight-for-age and length-for-age z-scores in infants with in-utero HIV and ART exposure: a prospective study in Cape Town, South Africa. BMC Pregnancy Childbirth. 2021;21(1):354.
le Roux SM, Jao J, Brittain K, Phillips TK, Olatunbosun S, Ronan A, et al. Tenofovir exposure in utero and linear growth in HIV-exposed, uninfected infants. AIDS. 2017;31(1):97–103.
Ejigu Y, Magnus JH, Sundby J, Magnus MC. Differences in growth of HIV-exposed uninfected infants in Ethiopia according to timing of in-utero antiretroviral therapy exposure. Pediatr Infect Dis J. 2020;39(8):730–6.
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection—recommendations for a public health approach—second edition. Geneva, Switzerland: World Health Organization.; 2016.
Wedderburn CJ, Evans C, Yeung S, Gibb DM, Donald KA, Prendergast AJ. Growth and neurodevelopment of HIV-exposed uninfected children: a conceptual framework. Curr HIV/AIDS Rep. 2019;16(6):501–13.
Ruel MT, Alderman H, Maternal Child Nutrition Study. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382(9891):536–51.
Keats EC, Das JK, Salam RA, Lassi ZS, Imdad A, Black RE, et al. Effective interventions to address maternal and child malnutrition: an update of the evidence. Lancet Child Adolesc Health. 2021;5(5):367–84.
Bekker A, Schaaf HS, Draper HR, Kriel M, Hesseling AC. Tuberculosis disease during pregnancy and treatment outcomes in HIV-infected and uninfected women at a referral hospital in Cape Town. PLoS ONE. 2016;11(11): e0164249.
World Health Organization, United Nations Children’s Fund, World Bank Group. Nurturing care for early childhood development: a framework for helping children survive and thrive to transform health and human potential. Geneva: World Health Organization; 2018.
Ramokolo V, Lombard C, Chhagan M, Engebretsen IM, Doherty T, Goga AE, et al. Effects of early feeding on growth velocity and overweight/obesity in a cohort of HIV unexposed South African infants and children. Int Breastfeed J. 2015;10:14.
Sartorius B, Sartorius K, Green R, Lutge E, Scheelbeek P, Tanser F, et al. Spatial-temporal trends and risk factors for undernutrition and obesity among children (<5 years) in South Africa, 2008–2017: findings from a nationally representative longitudinal panel survey. BMJ Open. 2020;10(4): e034476.
National Department of Health, Statistics South Africa, South African Medical Research Council, ICF. South Africa Demographic and Health Survey 2016. Pretoria, South Africa Rockville, Maryland, USA: NDoH, Stats SA, SAMRC, and ICF; 2019.
Hall K., Sambu W. Child hunger: Statistics South Africa (2003–2019) and General Household Survey (2002–2018): Children's Institute, University of Cape Town; 2019. http://childrencount.uct.ac.za/indicator.php?domain=4&indicator=32.
UNAIDS. New child infections due to gaps in prevention of vertical transmission 2020. http://aidsinfo.unaids.org/.
Sever JL, Rakusan TA, Campos JM, O’Donnell RM, Price VA. HIV antibody responses in children of HIV-infected mothers. Pediatr AIDS HIV Infect. 1996;7(4):246–53.
World Health Organization. WHO recommendations on the diagnosis of HIV infection in infants and children. Geneva: World Health Organization; 2010.
Tshivuila-Matala COO, Honeyman S, Nesbitt C, Kirtley S, Kennedy SH, Hemelaar J. Adverse perinatal outcomes associated with antiretroviral therapy regimens: systematic review and network meta-analysis. AIDS. 2020;34(11):1643–56.
Acknowledgements
We would like to thank the funders, the data collection teams and the women and children who participated in our study.
Disclaimer
The findings and conclusions in this manuscript are those of the author(s) and do not necessarily represent the official position of the funding agencies.
Funding
This publication was supported by funding from US President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention under terms of cooperative Agreement Number 1U2GPS001137 and 1U2GGH001150, UNICEF, the South African Medical Research Council, the South African National AIDS Council, the National Institute of Communicable Diseases, the National Department of Health and the Global Fund and L’Oreal-UNESCO For Women in Science South African National Young Talents Award.
Author information
Authors and Affiliations
Contributions
AEG, DJ and CL conceptualized and designed the primary study. AEG, DJ, CL and VR contributed to the implementation of the data collection. VR and LK conceptualized the analysis for the manuscript with input from CL, AEG and DJ. VR conducted the analysis with critical statistical input from LK and CL. VR, AEG, DJ, LK and CL contributed to the interpretation of the data. VR drafted and revised the manuscript drafts with. Critical input from LK, CL, AEG, and DJ. All authors accepted accountability for all aspects of the work. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We obtained ethical approval from the South African Medical Research Council institutional review board (protocol ID: EC09-002). This project was reviewed in accordance with CDC human research protection procedures and was determined to be research, but CDC investigators did not interact with participants or have access to identifiable data or specimens for research purposes. Participants provided written informed consent. Consent from a parent or legal guardian was obtained if the participant was a minor (aged < 18 years).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Box 1
. Anthropometry data cleaning criteria. Figure S1. Directed Acyclic Graph representing the hypothesized relationships, 2012–2014, South Africa. Figure S2. Study cohort profile of HIV exposed uninfected infants from 6-weeks to 18-months postpartum, 2012–2014, South Africa. Table S1. Proportion of underweight children from 6-weeks to 18-months postpartum by in-utero antiretroviral exposure status, 2012–2014, South Africa. Table S2. Proportion of stunted children from 6-weeks to 18-months postpartum by in-utero antiretroviral exposure status, 2012–2014, South Africa. Table S3. Frequency of maternal antiretroviral treatment over time by baseline maternal antiretroviral categories, 2012–2014, South Africa
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ramokolo, V., Kuhn, L., Lombard, C. et al. Impact of antenatal antiretroviral drug exposure on the growth of children who are HIV-exposed uninfected: the national South African Prevention of Mother to Child Evaluation cohort study. BMC Infect Dis 22, 908 (2022). https://doi.org/10.1186/s12879-022-07847-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07847-9