Abstract
Background
Increased occurrence of mucormycosis during the second wave of COVID-19 pandemic in early 2021 in India prompted us to undertake a multi-site case–control investigation. The objectives were to examine the monthly trend of COVID-19 Associated Mucormycosis (CAM) cases among in-patients and to identify factors associated with development of CAM.
Methods
Eleven study sites were involved across India; archived records since 1st January 2021 till 30th September 2021 were used for trend analysis. The cases and controls were enrolled during 15th June 2021 to 30th September 2021. Data were collected using a semi-structured questionnaire. Among 1211 enrolled participants, 336 were CAM cases and 875 were COVID-19 positive non-mucormycosis controls.
Results
CAM-case admissions reached their peak in May 2021 like a satellite epidemic after a month of in-patient admission peak recorded due to COVID-19. The odds of developing CAM increased with the history of working in a dusty environment (adjusted odds ratio; aOR 3.24, 95% CI 1.34, 7.82), diabetes mellitus (aOR: 31.83, 95% CI 13.96, 72.63), longer duration of hospital stay (aOR: 1.06, 95% CI 1.02, 1.11) and use of methylprednisolone (aOR: 2.71, 95% CI 1.37, 5.37) following adjustment for age, gender, occupation, education, type of houses used for living, requirement of ventilatory support and route of steroid administration. Higher proportion of CAM cases required supplemental oxygen compared to the controls; use of non-rebreather mask (NRBM) was associated as a protective factor against mucormycosis compared to face masks (aOR: 0.18, 95% CI 0.08, 0.41). Genomic sequencing of archived respiratory samples revealed similar occurrences of Delta and Delta derivates of SARS-CoV-2 infection in both cases and controls.
Conclusions
Appropriate management of hyperglycemia, judicious use of steroids and use of NRBM during oxygen supplementation among COVID-19 patients have the potential to reduce the risk of occurrence of mucormycosis. Avoiding exposure to dusty environment would add to such prevention efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic has adversely affected the world. As on 08th July 2022, globally, 558,703,551 confirmed cases and 6,369,057 deaths due to COVID-19 were reported [1]. The COVID-19 illness is further compounded by co-occurrence of fungal infections among patients with COVID-19, weeks or months after their recovery. Such occurrences of COVID-19 Associated Mucormycosis (CAM) were reported from several countries [2]. The unprecedented increase in cases of CAM in India during the second wave of the pandemic in May 2021 became a cause of concern and strained the already overwhelmed healthcare system [3]. By the end June 2021, 40,824 cases of mucormycosis had been reported from India with 3229 patients succumbing to death [4].
Mucormycosis is an opportunistic fungal infection caused by Mucorales. It is an invasive disease with protracted clinical course, challenging treatment options and a very high mortality rate, which has increased from 41% in pre-COVID-19 era to 49% during the pandemic [3, 5, 6]. Several equivocal hypotheses have been put forth regarding the risk factors associated with development of CAM. Systematic reviews from India and elsewhere have identified factors such as low oxygen milieu, diabetes mellitus (DM), inappropriate doses and duration of glucocorticoid use, host innate immunity related issues and prolonged duration of hospital stay with or without mechanical ventilation to be responsible for development of mucormycosis among COVID-19 patients [7]. A retrospective study from India before the second wave of the pandemic, during September-December 2020, revealed COVID-19 related hypoxemia and improper glucocorticoid use to be associated with CAM [8]. However, the surge of CAM cases during the second wave of the pandemic in India from May 2021 onward underlined the need for further in-depth investigation. Against this background, we conducted a multi-site case control investigation with the objectives of examining the monthly trend of proportion of CAM cases among in-patients since 1st January 2021 and identifying factors associated with the development of CAM post-second wave.
Material and methods
Study settings and participants
Study sites were the hospitals selected from the National Clinical Registry for COVID-19 as well as institutions associated with the Indian Council of Medical Research (ICMR) mycology research network [9]. Eleven sites were chosen across the country and grouped under four zones based on their geographic locations, namely: North, East, West and South plus Central. The sites were shortlisted based on a selection matrix, which included willingness to conduct the study, infrastructure for COVID-19 testing, capacity for diagnosis of fungal diseases and investigators’ research expertise. Each research team consisted of microbiologists and clinicians such as ophthalmologists, general physicians, otolaryngologists, and radiologists.
Trend analysis
Medical record-based analysis of monthly trend of confirmed cases of CAM was carried out in the participating hospitals during 1st January 2021 to 30th September 2021. The total number of patients admitted in the same hospitals were considered as denominator.
Cases and controls
A case was considered as CAM when a COVID-19 patient (active or recovered, any age and gender) was suspected of mucormycosis clinically and confirmed microbiologically for the same [10]. All specimens (either biopsy from paranasal sinus or nasal discharge or broncho-alveolar lavage or sputum) were considered microbiologically positive if aseptate or sparsely septate broad ribbon-like hyphae, with 90° branching angles were observed under direct microscopy in wet mount with potassium hydroxide (KOH) or lactophenol cotton blue or Periodic Acid Schiff (PAS) or Grocott-Gomori’s Methenamine-Silver (GMS) stain and/or culture on Sabouraud Dextrose Agar (SDA) showed cottony rapid growth with or without black heads, followed by identification of fungus by microscopy. Two controls were enrolled from a population of all COVID-19 patients discharged from the same hospital, 3–4 weeks prior to the date of diagnosis of an enrolled case of mucormycosis.
Suspected cases of mucormycosis, not microbiologically confirmed, irrespective of the treatment received and critically ill patients, unable to participate in the interview, were excluded from the study. The cases and controls were enrolled during 15th June 2021 to 30th September 2021. An individual once enrolled as control, was not considered in the population of controls for subsequent cases.
Sample size and sampling
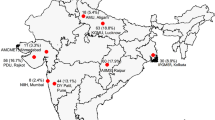
Based on previous reports, the least common risk factor for CAM was intensive care unit (ICU) stay. Taking the proportion of CAM and COVID-19 in-patients requiring ICU stay as 68% and 30%, respectively [11, 12] and a conservative Odds Ratio (OR) of 3, the sample size was estimated using Open Epi v3.0 (Rollins School of Public Health, Emory University) for unmatched case control design [13]. With an allocation ratio of 1:2, the sample size was calculated as 73 cases and 145 controls at the power of 95% and alpha error of 5%. In each zone (North, East, West and South plus Central), 218 patients (73 cases of CAM and 145 controls of COVID-19 without mucormycosis) were attempted to be enrolled with overall sample size of 872. Due to the dynamic situation of the pandemic and differential burden of disease during the study period, there was unequal enrolment from different geographic locations. The zone wise enrolment of cases and controls is depicted in the spot map presented as Additional file 1: Fig S1.
Study tools and data collection
Data were collected using a structured questionnaire with the following domains: (i) socio-demographic profile, (ii) present hospitalization, (iii) past hospitalization (in last 3 months), and (iv) home-based care for COVID-19. Data were extracted from the medical records of eligible cases and controls (as described above) by designated project staff posted at each of the study sites. They were trained by using virtual platform on how to use the paper-based clinical investigation reporting form (CRF) prior to data collection. The cases were enrolled while they were admitted for treatment at the hospital while the participants selected as controls were contacted telephonically. Their contact details and other pertinent clinical information were retrieved from hospital records. Confidentiality of the consenting participants was maintained, and patient identifiers were not entered in the CRF. They were requested to share their laboratory reports and treatment records on WhatsApp or via electronic mail (if not available from the hospital records).
Detection of variants through next generation sequencing
A subset of naso and oro-pharyngeal swab samples were transported to the ICMR-National Institute of Virology (ICMR-NIV), Pune in dry ice for next generation sequencing (NGS). Total RNA was extracted from 400 µl of each specimen using the Magmax™ Viral RNA/Pathogen RNA Extraction kit (Applied Biosystems, ThermoScientific, USA). Specimens with E gene Ct < 30 were processed for SARS-CoV-2 whole genome sequencing using the amplicon-based COVID Seq method (Illumina, USA) as per the instructions of the manufacturer. Total RNA was also tested for N and E subgenomic RNA (sgRNA). Viral gRNA and sgRNA were calculated using standard curve as described earlier [14]. NGS was performed using the Covidseq kits as described earlier [15].
Data management and analyses
Information collected on paper CRFs were entered in web portal designed by ICMR with unique login IDs for each participating site. Collation, validation, cleaning, and verification were carried out at the ICMR Headquarters. Chi-square test was used for trend analysis. Descriptive analyses of socio-demographic, clinical and laboratory profiles of all cases and controls were undertaken. Statistical analyses were performed using student ‘t’ or Mann Whitney U test as appropriate for continuous variables while categorical variables were compared using Chi-square or Fisher’s exact test. Probability at 5% level was considered as statistically significant. Multivariable logistic regression was used to identify factors associated with the development of CAM. Variables with significance at p value < 0.05 in bivariate analysis were included in logistic regression model. Doses of steroids such as dexamethasone, methylprednisolone and hydrocortisone were converted to equivalent doses of prednisolone and multiplied with the duration to obtain the administered cumulative dose. All statistical analyses were performed using R CRAN (version 4.0.2) software.
Ethical considerations
Approvals were obtained from the ICMR-Central Ethics Committee on Human Research and the Institutional Ethics Committee of each of the participating sites. Written informed consent was obtained from CAM cases while controls were contacted telephonically, and verbal consent was taken and recorded in the consent form by the interviewer. Parental consent was obtained for children aged less than 18 years. Verbal and written assent of child were obtained for children between 7 to 12 years and those above 12, respectively.
Results
Trend analysis of CAM cases and controls
Analysis of monthly trends revealed that CAM cases reached their peak during the month of May 2021 and then declined gradually till September 2021. The trend had an overall χ2 value of 5189.7 (p < 0.001). There was a lag of one month between the peak of COVID-19 cases admitted in hospitals and satellite peak of CAM cases as evident in Fig. 1.
Socio-demographic and clinical characteristics of CAM cases and controls
Of the 1235 patients screened for participation in this study from 11 sites, patients from one study site (n = 15) were excluded from analyses as they did not adhere to the criteria for case definition. Three more patients were excluded as microbiological confirmation of mucormycosis was not available and six were excluded because of non-availability of COVID-19 history. Thus, 1211 patients were enrolled in the study from 10 sites; 336 (27.7%) were CAM cases and 875 (72.3%) were COVID-19 positive non-mucormycosis controls. The study population was representative of four zones of the country namely, North (CAM: 24, 7.2%, COVID-19: 47, 5.4%), East (CAM: 30, 8.9%, COVID-19: 65, 7.4%), West (CAM: 119, 35.4%, COVID-19: 452, 51.7%), South plus Central (CAM: 163, 48.5%, COVID-19: 311, 35.5%). The details of the study enrollment are depicted in Fig. 2 and presented as a spot map in Additional file 1: Fig. S1.
The socio-demographic characteristics of cases and controls are depicted in Table 1. Nearly half of the CAM cases (157/336; 46.7%) belonged to 45 to 59-year age group, while 31.2% of the controls were in this age bracket. A significantly higher proportion of CAM cases were males (232/336; 69.1%). Most of the CAM and controls had studied till 12th standard (CAM: 185/336, 55.1%; Controls: 473/875, 54.1%). As compared to COVID-19 controls, a significantly higher proportion of CAM cases worked in dusty environment in daily life, being involved in either farming or gardening or both (n = 94, 27.9%) or working at construction sites (n = 19, 5.7%). A significantly higher proportion of CAM cases lived in cemented houses with thatched/asbestos roof or mud houses with thatched roof as compared to controls. The median (IQR) interval between COVID diagnosis and admission due to mucormycosis was 31 days (18, 47). The most frequent symptoms reported by CAM cases at the time of hospitalization due to mucormycosis were oro-facial symptoms namely toothache, facial swelling, or pain (n = 260, 77.4%) and ophthalmological symptoms like swelling, pain or redness in eye, blurry or double vision (n = 210, 62.5%) followed by lesser frequent symptoms such as headache, weakness, fever, cough, breathlessness, gastrointestinal or neurological symptoms. Symptom frequency of CAM cases at the time of hospitalization due to mucormycosis is presented in Additional file 2: Fig. S2.
Next generation sequencing and phylogenetic analysis
Next generation sequencing was performed on the samples from cases and controls having E gene Ct value < 30; samples from 29/43 (64.4%) CAM cases and 52/71 (73.2%) controls were sequenced. Complete genome could be retrieved from 19/29 (65.5%) CAM cases and 50/52 (96.2%) controls sequenced. The pangolin lineage for the genomic sequences with more than 98% retrieval was obtained [20 cases and 49 controls] using the online website (https://cov-lineages.org/resources/pangolin.html). Predominant SARS-CoV-2 lineages detected were B.1.617.2 [Delta variant] (CAM: n = 14 vs. Controls: n = 34), followed by AY.122 (CAM: n = 2 vs. Controls: n = 5) and AY.112 (CAM: n = 1 vs. Controls: n = 3). Frequencies of AY.44 and B.1.1.7 (Alpha variant) were equal in both CAM and controls (CAM: n = 1 vs. Controls: n = 1). Absence of AY.106, B.1.617.3 and AY.127, B.1.617.1 (Kappa variant) was observed in CAM cases. However, one sample each for former two and two samples each for latter two were observed in the control group. Details of the genomic reads mapped, total reads, pangolin lineage and the accession numbers for each of the strains retrieved are presented in Additional file 3: Table S1. A phylogenetic tree depicts a clear segregation of the clades (Fig. 3). The amino acid variation observed in the spike gene regions is depicted in Additional file 4: Fig. S3; this figure indicates the presence of similar mutations across the CAM cases and controls.

*Representative SARS-CoV-2 sequences from different lineages along with 81 sequences retrieved were used to generate the tree with a bootstrap replication of 1000 cycles. The retrieved pangolin lineages are marked on branches in different colors. The accession numbers of the retrieved sequences are highlighted in red. Fig Tree v1.4.4 and Inkscape were used to visualize and edit the generated tree
Maximum likelihood tree for the SARS-CoV-2 genomes retrieved in the study*.
Clinical and management profile of participants treated at hospitals due to COVID-19
The clinical, laboratory characteristics and management profile of the CAM cases were compared with that of controls as presented in Table 2. A higher proportion of CAM cases had headache (83; 36.7%) and central nervous system (CNS) symptoms (20; 8.8%) during their COVID-19 illness as compared to controls. Cases stayed for longer duration in hospitals [CAM: 9 days (6,12) vs. Controls: 7 days (4, 10), p < 0.001]. The COVID-19 patients who went on to develop CAM had a higher proportion of DM [(212; 87.6%) vs. (156; 17.9%)] as well as poorly controlled DM as evident through higher mean HbA1c (7.0 ± 2.8 vs. 5.9 ± 2.2, p < 0.001) in them. Participants who developed CAM later had a higher median level of random blood sugar at admission as well as higher maximum blood sugar level measured during hospital stay. It was intriguing that only a few patients of CAM had history of illnesses such as chronic obstructive pulmonary disease (n = 2, 0.2%) or cancer (4, 0.4%). Oxygen requirement was significantly higher in CAM cases as compared to COVID-19 controls [(144; 59.5%) vs. (446; 51.4%)]. The comparison of oxygen and steroid usage between CAM cases and controls are presented in Table 2.
Clinical and management profile of participants receiving home-based care after COVID-19 diagnosis
A small number of patients (n = 101) received exclusively home-based care for management of COVID -19. Of them, ninety-four patients developed mucormycosis later while seven did not. Of the 94 participants who developed mucormycosis later, only 15 (15.9%) required oxygen via oxygen cylinder for a median (IQR) duration of 5 days (4, 7). None of the participants used oxygen concentrators at home. The controls treated at home did not require oxygen and did not receive steroid during their course of illness, while 23 (24.5%) mucormycosis cases received either methylprednisolone (n = 12; 52.2%) or dexamethasone (n = 11; 47.8%) for management of COVID -19 at home. It was also observed that higher proportion of patients hospitalized for management of COVID -19 developed CAM as compared to those treated at home (p < 0.001).
Multivariable model
In multivariable analysis, adjusting for age, gender, occupation, education, residence type and various treatment related issues, five factors were identified to be independently associated with CAM (Table 3). These included history of working in dusty environments, duration of hospital stay during COVID-19 illness, presence of DM, mode of oxygen supplementation and receipt of methylprednisolone. While the odds of occurrence of CAM was about three times in individuals working in dusty environments or those who had received methylprednisolone, it was higher at 32 for those who had DM. Longer duration of hospital-stay for COVID-19 management had marginal effect on increasing the odds of developing CAM. Use of non-rebreather mask (NRBM) for oxygen supplementation was protective against development of CAM as compared to the use of face masks (Table 3).
Discussion
The present multi-site, nationwide study clearly depicted that the trend of CAM cases in hospitals in India peaked during the month of May 2021, about a month following the peak in admission of COVID-19 cases in April of the same year. This could probably be explained by complex immune response due to SARS-CoV-2 infection leading to a stage of immunosuppression after the initial cytokine storm, particularly during the second week of infection [16, 17]. Immunosuppression due to COVID-19 associated treatment with steroid could have further compounded this phenomenon. Noticeably, a similar occurrence of satellite epidemic of herpes zoster was recorded in the early 1990s following HIV epidemic induced immunosuppression among young injection heroin users in the north-eastern state of Manipur bordering Myanmar [18].
The socio-demographic profile of CAM cases in our study were similar to that in other Indian studies from Chandigarh, Delhi and Pune, and an online Mycotic Infections in COVID-19 (MUNCO) registry [8, 19,20,21,22]. Saprophytic fungi such as Mucorales are found in ecological spaces such as soil, dust and decomposing vegetation [23]. The results of the current study corroborated with this fact as more than one-fourth of the CAM cases (27.9%) reported dusty working environments such as either farming or gardening or both, whereas frequency of such occupational exposure among controls was only 14.7%.
Genomic sequencing of cases and controls showed a comparable presence of SARS-CoV-2 variants and did not reveal any specific association of mutation in either group. Variants of concern (Alpha, Delta and Delta derivates) or variants under investigation did not show any preferential distribution among CAM cases. However, the small sample size used in genomic sequencing precludes us from drawing any further inference from the same.
Uncontrolled DM is a known risk factor for mucormycosis. A systematic review of CAM cases globally, revealed diabetes to be a more frequently reported association from India than elsewhere (66.1% vs. 54.8%) [24]. DM as a risk factor has been reported consistently from studies conducted across India during COVID-19 pandemic [5, 19, 21, 22, 25]. There is emerging evidence of hyperglycemia induced increase in surface glucose-regulated protein (GRP78) expression on the endothelium, which in turn not only facilitates SARS CoV-2 entry by forming a complex with spike protein and angiotensin converting enzyme 2 (ACE2) receptors, but also mediates interaction with Mucorales spores through spore protein homologue CotH3 and promotes endothelial invasion [26, 27]. It is therefore important to monitor blood glucose levels during COVID-19 management and attain good glycemic control, with special attention on severe cases of COVID-19 who are on systemic corticosteroids.
Evidence is equivocal with respect to the role of supplemental oxygen during COVID-19 illness and subsequent development of CAM. While the current study and other studies on CAM showed higher requirement of oxygen among CAM cases during COVID illness [8, 28], studies conducted in other Indian cities such as Delhi and Pune did not witness such association [19, 21]. Interestingly, use of NRBM was found to be a protective factor against development of CAM in the current investigation. Use of high flow oxygen devices was reported to be lower in CAM group in study conducted in Delhi, India [19]. Moreover, unhygienic ways to deliver oxygen or prolonged use of same mask for more than two patients has been hypothesized for occurrence of mucormycosis [3].
Irrational corticosteroid use has been associated with development of many opportunistic infections including mucormycosis. Steroids induce immunosuppression by inhibiting macrophages and neutrophils and raise blood sugar levels, thereby increasing the risk of CAM. Inappropriate use of steroids during the second wave of COVID-19 pandemic could have resulted in prolonged hyperglycemia among pre-diabetic and diabetic patients, which in turn probably resulted in invasive mucormycosis. Corticosteroid use, one of the proven predisposing factors for mucormycosis, has not been recognized as an independent risk factor in the current study [29]. However, we observed that the odds of developing CAM were nearly three times higher with the use of methylprednisolone as compared to dexamethasone. Though efficacy of both dexamethasone and methylprednisolone is comparable, preclinical studies have demonstrated a higher lung to plasma ratio for methylprednisolone compared with dexamethasone [30]. We may hypothesize that this led to more severe immunosuppression, rendering the lung tissue incapable of getting rid of the fungal spores.
Strengths and limitations of the study
The current investigation was a large, multi-site study conducted across four regions of the country. Validated methods were used to ascertain cases and controls. However, the current study had a few limitations as well. The information related to laboratory parameters and treatment was retrieved from paper-based medical records, which were not uniformly available across all institutes, hence not included in the multivariate model. Due to the nature of data collection (i.e., telephonically for controls), detailed information on dusty environments, use of alternative medicines or over-the-counter purchase and usage of steroids could not be explored in the current investigation. Moreover, recall bias cannot be ruled out regarding information obtained from controls. Lastly, we did not explore the impact of climate of a place and hospital environment related factors (e.g., humidifiers in the ICUs) which could serve as a potential source for mucormycosis outbreaks [31, 32]. However, this objective was beyond the scope of our investigation.
To conclude, CAM was found to be strongly associated with host factors such as diabetes mellitus and environmental factors such as working in dusty environment. Factors related to clinical management such as duration of hospital stay during COVID -19 illness and use of steroids increased the odds of CAM. On the other hand, oxygen supplementation through NRBM had a protective effect. Appropriate management of hyperglycemia, judicious use of steroids and use of NRBM during oxygen supplementation among COVID-19 patients thus emerged as potential intervention areas to prevent subsequent occurrence of mucormycosis.
Availability of data and materials
The next generation sequencing data generated during the current study are available in the GISAID database, (GISAID-Initiative https://www.gisaid.org/).
References
Worldometer. COVID-19 Coronavirus Pandemic. https://www.worldometers.info/coronavirus/. Accessed 8 Jul 2022.
Rodriguez-Morales AJ, Sah R, Millan-Oñate J, Gonzalez A, Montenegro-Idrogo JJ, Scherger S, et al. COVID-19 associated mucormycosis: the urgent need to reconsider the indiscriminate use of immunosuppressive drugs. Ther Adv Infect Dis. 2021;8:20499361211027064.
Aranjani JM, Manuel A, Abdul Razack HI, Mathew ST. COVID-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS Negl Trop Dis. 2021;15: e0009921.
National Centre for Disease Control. CD Alert: Covid-19 Associated Mucormycosis. https://ncdc.gov.in/WriteReadData/l892s/20590398661627904360.pdf. Accessed 25 Dec 2021.
Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux J-P, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022;3:e543–52.
Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Chen SC-A, et al. Contemporary management and clinical outcomes of mucormycosis: a systematic review and meta-analysis of case reports. Int J Antimicrob Agents. 2019;53:589–97.
Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15: 102146.
Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, et al. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis. India Emerg Infect Dis. 2021;27:2349–59.
Kumar G, Mukherjee A, Sharma RK, Menon GR, Sahu D, Wig N, et al. Clinical profile of hospitalized COVID-19 patients in first & second wave of the pandemic: insights from an Indian registry based observational study. Indian J Med Res. 2021;153:619–28.
Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–21.
John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi (Basel). 2021;7:298.
Budhiraja S, Soni A, Jha V, Indrayan A, Dewan A, Singh O, et al. Clinical Profile of First 1000 COVID-19 Cases Admitted at Tertiary Care Hospitals and the Correlates of their Mortality: An Indian Experience. MedRxiv. 2020.
Dean A, Sullivan K, Soe M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. 3.0.
Yadav PD, Ella R, Kumar S, Patil DR, Mohandas S, Shete AM, et al. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat Commun. 2021;12:1386.
Gupta N, Kaur H, Yadav PD, Mukhopadhyay L, Sahay RR, Kumar A, et al. Clinical characterization and genomic analysis of samples from COVID-19 breakthrough infections during the second wave among the various States of India. Viruses. 2021;13:1782.
Samson R, Dharne M. COVID-19 associated mucormycosis: evolving technologies for early and rapid diagnosis. 3 Biotech. 2022;12:6.
Reusch N, De Domenico E, Bonaguro L, Schulte-Schrepping J, Baßler K, Schultze JL, et al. Neutrophils in COVID-19. Front Immunol. 2021;12: 652470.
Panda S, Sarkar S, Mandal BK, Singh TB, Singh KL, Mitra DK, et al. Epidemic of herpes zoster following HIV epidemic in Manipur. India J Infect. 1994;28:167–73.
Arora U, Priyadarshi M, Katiyar V, Soneja M, Garg P, Gupta I, et al. Risk factors for Coronavirus disease-associated mucormycosis. J Infect. 2022;84:383–90.
Meher R, Wadhwa V, Kumar V, Shisha Phanbuh D, Sharma R, Singh I, et al. COVID associated mucormycosis: a preliminary study from a dedicated COVID Hospital in Delhi. Am J Otolaryngol. 2022;43: 103220.
Gupta D, Kulkarni R, Pujari S, Mulay A. Covid-19 Associated mucormycosis: a case-control study. MedRxiv. 2021.
Arora S, Hemmige VS, Mandke C, Chansoria M, Rawat SK, Dravid A, et al. Online registry of COVID-19-associated mucormycosis cases, India, 2021. Emerg Infect Dis. 2021;27:2963–5.
Richardson MD, Rautemaa-Richardson R. Biotic environments supporting the persistence of clinically relevant mucormycetes. J Fungi. 2019;6:4.
Muthu V, Rudramurthy SM, Chakrabarti A, Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186:739–54.
Gupta R, Kesavadev J, Krishnan G, Agarwal S, Saboo B, Shah M, et al. COVID-19 associated mucormycosis: a descriptive multisite study from India. Diabetes Metab Syndr. 2021;15: 102322.
Carlos AJ, Ha DP, Yeh D-W, Van Krieken R, Tseng C-C, Zhang P, et al. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. J Biol Chem. 2021;296: 100759.
Alqarihi A, Gebremariam T, Gu Y, Swidergall M, Alkhazraji S, Soliman SSM, et al. GRP78 and integrins play different roles in host cell invasion during mucormycosis. MBio. 2020;11:e01087-e1120.
Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U, et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India—Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69:1670–92.
Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34.
Annane D, Pastores SM, Arlt W, Balk RA, Beishuizen A, Briegel J, et al. Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med. 2017;43:1781–92.
Hartnett KP, Jackson BR, Perkins KM, Glowicz J, Kerins JL, Black SR, et al. A guide to investigating suspected outbreaks of mucormycosis in healthcare. J Fungi. 2019;5:69.
Gadre A, Enbiale W, Andersen LK, Coates SJ. The effects of climate change on fungal diseases with cutaneous manifestations: a report from the International Society of Dermatology Climate Change Committee. J Clim Change Health. 2022;6: 100156.
Acknowledgements
Authors are grateful for the excellent support provided by Dr. Abhinendra Kumar, Mrs. Savita Patil, Ms. Manisha Dudhmal, Mr. Yash Joshi, Mr. Ajay Kumar, Mrs. Kaumudi Kalele, Ms. Pranita Gawande, Ms. Jyoti Yemul for processing of the samples for NGS at ICMR-NIV, Pune.
CONSORTIUM NAME: ICMR- Mucormycosis group
Anita M. Shete3, Triparna Majumdar3, Priya Abraham3, Anudita Bhargava4, Rupa Mehata4, Ripu Daman Arora4, Richa Tigga4, Gopa Banerjee5, Vijay Sonkar5, HS Malhotra5, Neeraj Kumar5, Rajashri Patil7, Chandrashekhar G Raut7, Kumkum Bhattacharyya8, Preetam Arthur9, L Somu9, Padma Srikanth9, Naresh K Panda10, Dipti Sharma10, Wasil Hasan11, Aftab Ahmed11, Meeta Bathla13, Sunita Solanki13, Hiren Doshi13, Yash Kanani12, Nishi Patel13, Zincal Shah13, Alok Kumar Tembhurne14, Chhaya Rajguru (Waghmare)16, Lalitkumar R Sankhe16, Shrinivas S Chavan16, Reetika Malik Yadav14, Vikas Deswal15, Kuldeep Kumar15
3ICMR-National Institute of Virology, Pune, India. 4All India Institute of Medical Sciences, Raipur, India, 5King George's Medical University, Lucknow, India. 7Dr. D. Y. Patil Medical College, Hospital and Research Centre, Pune, India. 8Institute of Post Graduate Medical Education and Research, Kolkata, India. 9Sri Ramachandra Medical College and Research Institute, Chennai, India. 10Post Graduate Institute of Medical Education and Research, Chandigarh, India. 11Jawaharlal Nehru Medical College Aligarh Muslim University, Aligarh, India. 12Smt. NHL Municipal Medical College, Ahmedabad, India. 13AMC MET Medical College, Ahmedabad, India. 14ICMR-NIIH, Mumbai, India. 15Medanta-The Medicity, Gurugram, India. 16Grant Government Medical College and Sir J.J Group of Hospitals, Mumbai, India.
Funding
Indian Council of Medical Research, New Delhi, India.
Author information
Authors and Affiliations
Consortia
Contributions
TA, AM, SPa, KJS and MD conceptualized and designed the study. AYK, NMN, PG, DH, SNM, JDP, PR, SR, SG, AH, AJK, RA, SMR, MPS, MS, NF, JRK, SP, MM, VDP, SK, PS implemented and supervised the study at their institutes. All authors provided administrative, material and technical support. TA, KJS, PSV and AS coordinated and monitored data collection at the sites. PSV, AS and KJ performed data cleaning and management. KJS, AM, AS and KJ conducted data analysis. TA wrote the first draft of the manuscript with inputs from AM, AS and KJS and critical review by SPa. PDY and RRS- conducted genomic analysis at NIV-Pune and drafted corresponding part in the manuscript. TA, AM, AS and KJS made substantial revisions to the manuscript. All members of the ICMR- Mucormycosis group provided inputs in drafting and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approvals were obtained from the ICMR-Central Ethics Committee on Human Research and the Institutional Ethics Committee of each of the participating sites. Informed consent was obtained from all the participants. The study was performed in accordance with the principles of Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Figure S1: Geographic distribution of CAM cases (n=336) and COVID-19 controls (n = 875)**. **The values for CAM cases and COVID-19 controls are representative of each of the four zones i.e., North, East, West and South plus Central.
Additional file 2.
Figure S2: Symptom frequency of CAM cases (n = 336).
Additional file 3.
Table S1: Details of the genomic reads mapped, total reads, pangolin lineage and the accession numbers for each of the strains retrieved.
Additional file 4.
Figure S3: Single nucleotide variation (SNV) of three SARS-CoV-2 strains***. ***Single nucleotide variation of strain in the spike protein region. The X-axis shows the nucleotide mutation at the specified gene location and the Y-axis depicts the case analyzed (C: COVID-19; and M: COVID-19 infected with mucormycosis). The amino acid mutations caused due to the SNVs is shown at the top of the x- axis. The frequency of reads observed for the specific SNV is depicted using different color (maximum: red; minimum: green;) color. Dashed line indicates the segregation of cases.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Anand, T., Mukherjee, A., Satija, A. et al. A case control investigation of COVID-19 associated mucormycosis in India. BMC Infect Dis 22, 856 (2022). https://doi.org/10.1186/s12879-022-07844-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07844-y