Abstract
Objective
To study the risk factors and prediction models of multidrug resistance in patients with tuberculosis and diabetes and those with a history of tuberculosis treatment.
Methods
A total of 256 tuberculosis patients with diabetes who were registered in Luoyang city, Henan Province, from January 2018 to December 2021. Logistic regression analysis was performed to analyse the risk factors for multidrug resistance. ROC curves were used to analyse the predictive model for multidrug resistance.
Results
Age < 65 years old, HbA1c, and a history of tuberculosis treatment were independent risk factors for multidrug resistance in patients with tuberculosis and diabetes (P < 0.05). The area under the ROC curve of predictive model for MDR was 0.878 (95% CI (0.824, 0.932)). Age < 65 years old and HbA1c were independent risk factors for MDR in patients with TB and diabetes with a history of TB treatment. The area under the ROC curve of predictive model for MDR was 0.920 [95% CI (0.831, 0.999)].
Conclusion
The predictive model had certain prediction value for the risk of multidrug resistance in patients with tuberculosis and diabetes.
Similar content being viewed by others
Background
Tuberculosis (TB) is one of the deadliest infectious diseases in the world. Despite a slow decline in its incidence, tuberculosis remains a major global public health problem. The growing prevalence of diabetes mellitus (DM) has emerged as one of the major global health challenges and may lead to an increase in the burden of TB. China has a heavy burden of TB with DM (patients had both tuberculosis and diabetes) [1]. Studies have shown that due to the impaired immune function of DM patients, the risk of active TB increases by 3 times [2]. Approximately 15% of TB cases worldwide are attributed to DM [3], and approximately 17% of TB cases in China are attributed to DM [4]. DM also leads to the occurrence of multidrug-resistant tuberculosis (MDR TB) to a certain extent [5], which is positively correlated with the occurrence of MDR TB [6]. MDR TB is defined as tuberculosis that is resistant to at least both rifampicin and isoniazid [7]. The diagnosis and treatment of MDR TB is an ongoing global public health challenge [8]. Early identification of risk factors for MDR TB in patients with TB and DM could reduce the risk of MDR TB and can simultaneously facilitate early diagnosis and treatment of patients. Studies have reported that in global the incidence rates of multidrug resistance in newly diagnosed and previously treated TB patients are 3.6% and 17%, respectively [9]. In China, the incidence rates of multidrug resistance in newly diagnosed and previously treated TB patients are 7.1% and 24% [10]. A history of TB treatment is an independent risk factor for the occurrence of multidrug resistance, therefore, patients with a history of TB treatment warrant greater attention. The main purpose of this study was to study the risk factors and predictive models of multidrug resistance in patients with TB and DM and those with a history of TB treatment.
Methods
Patient selection
The study was carried out as a nationwide retrospective register-based cohort study including all adult patients (> 18 years) notifed with TB and DM (including T1DM and T2DM) in Luoyang city, Henan Province, from January 2018 to December 2021. Both patients with pulmonary TB (PTB) and extrapulmonary TB (ETB) were included in the study. Inclusion criteria: Patients diagnosed with TB and DM. Inclusion criteria of TB dependedon the Chinese guidelines for the diagnosis and treatment of TB (2020 version) [11]: (1) The sputum smear was positive for acid-fast bacilli; (2) Mycobacterium tuberculosis cogroup was cultured in sputum, bronchoalveolar lavage fluid or pleural effusion; (3) The nucleic acid of Mtb and/or Mtb culture was positive in sputum, bronchoalveolar lavage fluid, or pleural effusion; (4) the pathological staining of lung tissue specimens was positive for acid-fast bacilli, or the nucleic acid of Mtb was positive in lung tissue specimens from the lesion site. The diagnosis of pulmonary TB could be clarified if any of the above four items were met. The patient was diagnosed with MDR TB by sputum culture and DST, GeneXpert MTB/RIF1, or only sputum culture and DST demonstrated at least simultaneous resistance to isoniazid and rifampicin. Diagnostic criteria for DM: (1) FPG ≥ 7.0 mmol/L (126 mg/dL); (2) A 2-h plasma glucose level of 200 mg/dL (11.1 mmol/L) or higher during OGTT (the 75-g oral glucose tolerance test) or HbA1c ≥ 6.5% [12, 13]. Exclusion criteria: Patients without sputum culture and drug sensitivity test; patients with an unclear diagnosis of DM, and patients with incomplete data.
Data collection
The following clinical data of patients were collected: sex, age, body mass index (BMI), smoking history, history of alcoholism, history of TB treatment (including a end of the original treatment course, treatment course interruption, treatment failure, using a non-standard TB regimen), DM duration, co-morbidities (chronic obstructive pulmonary disease, coronary heart disease, hypertension, cerebral infarction, HIV), treatment options for diabetes, tuberculous pleuritis, Chest CT showed cavity, laboratory indices (HbA1c, hypersensitive c-reactive protein, erythrocyte sedimentation rate, white blood cell count, neutrophils, haemoglobin, platelets, glutamic-pyruvic transaminase (ALT), glutamic oxaloacetic transaminase(AST), albumin, uric acid, creatinine). The above data are the data of patients at the time of registration.
Statistical analysis
SPSS 23.0 and MedCalc were used for statistical analysis. Means were compared using an independent t test. Count data were expressed as percentages, and differences in these characteristics between cases and controls were analysed using the chi-square test. Risk factors were analysed by multivariable logistic regression analysis. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were used to evaluate the predictive value of each risk factor and its combination in the occurrence of multidrug resistance in TB with DM. A p-value < 0.05 was considered statistically significant.
Results
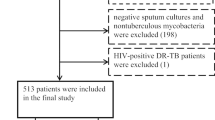
A total of 256 patients were registered with TB with DM. According to the exclusion criteria, 200 patients with TB and DM were included, including 156 non-MDR TB patients and 44 MDR TB patients. Among the 200 patients with TB and DM, 44 patients had a history of TB treatment, including 18 patients with non-MDR TB and DM and 26 patients with MDR TB and DM. 44 patients of had a treatment history of TB including 23 cases had a end of the original treatment course, 8 cases had a treatment course interruption, 10 cases had a treatment failure, 3 cases had a non-standard TB regimen. The inclusion flow chart is shown in Fig. 1.
Comparison of general data between patients with non-MDR TB and DM and patients with MDR TB and DM
Previous studies showed that MDR-TB patients were more likely to be younger than 65 years old [14,15,16,17], so we put the age cut-off at 65 years old. As shown in Table 1, the percentage of patients with MDR TB and DM was significantly higher than the non-MDR TB and DM in aged < 65 years old (p < 0.05), the percentage of MDR TB and DM patients with a smoking history and TB treatment history were significantly higher than non-MDR TB and DM (p < 0.05), and the level of HbA1c of MDR TB and DM was significantly higher than non-MDR TB and DM (p < 0.05).
Risk factors for multidrug resistance in TB and DM
Age < 65 years old, smoking history, a history of TB treatment, HbA1c and sex were entered into a multivariable logistic regression analysis. Age < 65 years old, HbA1c level and a history of TB treatment were independent risk factors for multidrug resistance in patients with TB and DM (p < 0.05), as shown in Table 2.
Predictive value of age < 65 years old, HbA1c, a history of TB treatment, and the three risk factors combined for multidrug resistance in patients with TB and DM
ROC curves for the predictive value of age < 65 years old, HbA1c, a history of tuberculosis treatment and the three risk factors combined for multidrug resistance in TB and DM were drawn (Fig. 2). The AUC of age < 65 years old was 0.624 (95% CI (0.536, 0.712)), the sensitivity was 81.82%, and the specificity was 42.95%. The AUC of HbA1c was 0.739 (95% CI (0.666, 0.812)), and the sensitivity and specificity were 77.27% and 61.54%, respectively, when the optimal cut-off value was 9.3%. The AUC of TB treatment history was 0.741 (95% CI (0.648, 0.834)), the sensitivity was 59.09%, and the specificity was 89.1%. The AUC of the combination of age < 65 years old, HbA1c and a tuberculosis treatment history was 0.878 (95% CI (0.824, 0.932)), the sensitivity was 63.64%, and the specificity was 95.91%.
Comparison of general data between patients with non-MDR TB and DM and patients with MDR TB and DM with a history of TB treatment
As shown in Table 3, the percentage of MDR TB and DM was significantly higher than non-MDR TB in aged < 65 years old (p < 0.05), and HbA1c of MDR TB patients was significantly higher than non-MDR TB (p < 0.05).
Risk factors for multidrug resistance in patients with TB and DM with a history of TB treatment
Age < 65 years old, HbA1c and sex were entered into a multivariable logistic regression analysis. Age < 65 years old and HbA1c were independent risk factors for multidrug resistance in patients with TB and DM with a history of TB treatment (p < 0.05), as shown in Table 4.
Predictive value of age < 65 years old, HbA1c, and the two risk factors combined for multidrug resistance in TB and DM
ROC curves of the predictive value of age < 65 years old, HbA1c, and the combination of the two risk factors for multidrug resistance in patients with TB and DM with a history of TB treatment were constructed (Fig. 3). The AUC of age < 65 years old was 0.757 (95% CI (0.601, 0.912)), the sensitivity was 80.77%, and the specificity was 70.59%. The AUC of HbA1c was 0.812 (95% CI (0.687, 0.938)), the sensitivity and specificity were 61.54% and 94.12%, respectively, when the optimal cut-off value was 9.9%. The AUC of age < 65 years old and HbA1c combined was 0.920 (95% CI (0.831, 0.999)), the sensitivity was 92.31%, and the specificity was 88.24%,
Discussion
The global burden of combined TB-DM is high, with a global prevalence of 16% and rates of 24% in North America, 23% in Oceania, 17% in Asia, 11% in South America, 7% in Africa and 6% in Europe [3]. Diabetes may lead to immune dysfunction and changes in cytokine levels and the activation status of macrophages. Meanwhile, a high-glucose acidic environment may be conducive to the growth of MDR TB, which may reduce the efficacy of anti-tuberculosis drugs and increase the risk of drug resistance [18,19,20]. MDR TB is difficult to treat and has a high mortality rate. Some studies have shown that 20% of MDR TB patients died during treatment [21]. Previous studies had focused more on risk factors for MDR-TB. However, few studies had been conducted on the risk factors for multidrug resistance in TB and DM. Early identification of risk factors for MDR TB in patients with TB and DM could reduce the risk of MDR TB and can simultaneously facilitate early diagnosis and treatment of patients.
Former studies had shown that alcoholism, smoking, irregular treatment and lung cavities, age under 65 years, diabetes and HIV positivity may be risk factors for MDR-TB [10, 22]. Bocar Baya [24] concluded age < 65 years, previous TB treatment failures, number of previous TB treatment courses < 2, close contact with TB patients were risk factors for MDR-TB. However, few studies have been conducted on the risk factors for multidrug resistance in TB and DM. In our study, the HbA1c level was an independent risk factor for multidrug resistance in TB and DM. In our study, the prediction value of multidrug resistance was higher when HbA1c was 9.3%. Mengyuan Lyu [24] have concluded that HbA1c grade of 7% has higher predictive value for multidrug resistance. The reasons are as follows: a high HbA1c level is considered a factor leading to tissue hypoxia by increasing the affinity of haemoglobin for oxygen, while a low tissue oxygen concentration can lead to oxidative stress. An increased HbA1c may lead to resistance to isoniazid by contributing to oxidative stress [25]. Hyperglycaemia can reduce the concentration of anti-tuberculosis drugs in the blood by delaying drug absorption and promoting drug clearance, and the reduction in the drug concentration is conducive to the development of drug resistance to a large extent [20]. There were meta-analyses showed that MDR-TB patients were more likely to be younger than 65 years old [17]. A study had shown that aged between 45 and 64 years was risk factors for acquired drug resistance among retreated TB-DM cases, 65 ≥ years old was protective factors for acquisition of drug resistance [14]. Our study concluded that age < 65 years old was an independent risk factor for multidrug resistance in patients with TB and DM, which was consistent with FAUSTINI A et al. [17].The considered reason was that MDR-TB patients were younger than non-MDR-TB patients [23]. Previous studies had found that MDR-TB was more common in patients younger than 65 years of age given that they were busier with work or study than older patients and subsequently more exposed to the risk of MDR-TB [15, 16]..
There were two mechanisms for DR-TB: (i) acquired (or secondary) resistance: Mycobacterium tuberculosis strains develop resistance during TB treatment, and acquired resistance was defined as occurring when baseline DST result showed susceptibility in vitro, the final DST result showed resistance in vitro. (ii) transmitted (or primary) resistance: infection with a drug-resistant strain [14] .It had been widely accepted that had an inadequate treatment history were the major reasons of acquired drug resistance in TB patients [14]. Among the 44 MDR TB with DM patients in our study, 26 had a history of TB treatment, 26 were considered as acquired MDR and 18 were primary MDR. Prior treatment had been one of the most consistent independent risk factors for MDR-TB [23]. Our study found that a history of TB treatment was an independent risk factor for drug resistance in patients with TB and DM. As TB patients with a history of TB treatment had a significantly increased probability of developing multidrug resistance, some studies in China had shown that the incidence of MDR-TB in re-treated tuberculosis patients was 26.3% (23.1%–29.7%) [14]. However, few previous study had focused on the risk factors for MDR-TB in patients with TB and DM with a history of TB treatment separately. In our study, risk factors for multidrug resistance in patients with TB and DM with a history of TB treatment were analyzed separately, age < 65 years old and HbA1c remained independent risk factors for multidrug resistance.
Mengyuan Lyu [24] concluded that the AUC of modle of HbA1c rating (7%), age, erythrocyte sedimentation rate, hemoglobin, and C-reactive protein predicting MDR TB was 0.754, had a better predictive value. In our study, combining age < 65 years old, HbA1c and a TB treatment history yielded a certain predictive value for multidrug resistance in patients with TB and DM, the AUC was 0.878. Combining age < 65 years old and HbA1c yielded higher predictive value for multidrug resistance in patients with TB and DM with a history of TB treatment, the AUC was 0.920.
Limitations
First, we separately analyzed the risk factors for MDR in patients with TB and DM who had a history of TB treatment, however due to the small number of patients with primary MDR, we did not analyze the risk factors for primary MDR separately. Second, although we had collected all TB-DM cases with susceptibility data and information in Luoyang city, Henan Province, from January 2018 to December 2021, the sample size was still limited. More appropriate cases need to be enrolled in order to gain a higher validity and reliability of our findings.
Conclusion
Our study concluded that age < 65 years old, HbA1c and a history of TB treatment were independent risk factors for MDR in patients with TB and DM. The combination of the above three risk factors had certain predictive value for multidrug resistance in patients with TB and DM. Age < 65 years old and HbA1c were independent risk factors for multidrug resistance in patients with TB and DM who had a history of TB treatment. The combination of the above two risk factors had high predictive value for the risk of multidrug use in patients with TB and DM who had a history of TB treatment. The predictive model had certain prediction value for the risk of multidrug resistance in patients with tuberculosis and diabetes.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Li Y, Guo J, Xia T, et al. Incidence of pulmonary tuberculosis in Chinese adults with type 2 diabetes: a retrospective cohort study in Shanghai. Sci Rep. 2020;10(1):8578.
Lutfiana NC, van Boven J, Masoom ZM, et al. Diabetes mellitus comorbidity in patients enrolled in tuberculosis drug efficacy trials around the world: a systematic review. Br J Clin Pharmacol. 2019;85(7):1407–17.
Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: a systematic review. PLoS ONE. 2017;12(4): e175925.
van Crevel R, van de Vijver S, Moore D. The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol. 2017;5(6):457–68.
Desissa F, Workineh T, Beyene T. Risk factors for the occurrence of multidrug-resistant tuberculosis among patients undergoing multidrug-resistant tuberculosis treatment in East Shoa, Ethiopia. BMC Public Health. 2018;18(1):422.
Chang JT, Dou HY, Yen CL, et al. Effect of type 2 diabetes mellitus on the clinical severity and treatment outcome in patients with pulmonary tuberculosis: a potential role in the emergence of multidrug-resistance. J Formos Med Assoc. 2011;110(6):372–81.
Mirzayev F, Viney K, Linh NN, et al. World Health Organization recommendations on the treatment of drug-resistant tuberculosis, 2020 update. Eur Respir J. 2021;57:6.
Tong X, Wang D, Wang H, et al. Clinical features in pulmonary tuberculosis patients combined with diabetes mellitus in China: An observational study. Clin Respir J. 2021;15(9):1012–8.
WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization, 2019.
Duan Q, Chen Z, Chen C, et al. The Prevalence of Drug-Resistant Tuberculosis in Mainland China: An Updated Systematic Review and Meta-Analysis. PLoS ONE. 2016;11(2): e148041.
Jin F, Li Q, Bai C, et al. Chinese expert recommendation for diagnosis and treatment of massive hemoptysis. Respiration. 2020;99(1):83–92.
Diagnosis and classification of diabetes mellitus. Diabetes Care, 2014,37 Suppl 1: S81-S90.
Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva: World Health Organization, 2011.
Song WM, Li YF, Liu JY, et al. Drug resistance of previously treated tuberculosis patients with diabetes mellitus in Shandong, China. Respir Med. 2020;163: 105897.
Hameed S, Ahmad SR, Rahman M, et al. Drug resistance profile of Mycobacterium tuberculosis and predictors associated with the development of drug resistance. J Glob Antimicrob Resist. 2019;18:155–9.
Soeroto AY, Pratiwi C, Santoso P, et al. Factors affecting outcome of longer regimen multidrug-resistant tuberculosis treatment in West Java Indonesia: a retrospective cohort study. PLoS ONE. 2021;16(2): e246284.
Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61(2):158–63.
Kumar NP, Babu S. Influence of diabetes mellitus on immunity to human tuberculosis. Immunology. 2017;152(1):13–24.
Lopez-Lopez N, Martinez A, Garcia-Hernandez MH, et al. Type-2 diabetes alters the basal phenotype of human macrophages and diminishes their capacity to respond, internalise, and control Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz. 2018;113(4): e170326.
Huang D, Wang Y, Wang Y, et al. The impact of diabetes mellitus on drug resistance in patients with newly diagnosed tuberculosis: a systematic review and meta-analysis. Ann Palliat Med. 2020;9(2):152–62.
Kizito E, Musaazi J, Mutesasira K, et al. Risk factors for mortality among patients diagnosed with multi-drug resistant tuberculosis in Uganda- a case-control study. BMC Infect Dis. 2021;21(1):292.
Smith SE, Ershova J, Vlasova N, et al. Risk factors for acquisition of drug resistance during multidrug-resistant tuberculosis treatment, Arkhangelsk Oblast, Russia, 2005–2010. Emerg Infect Dis. 2015;21(6):1002–11.
Baya B, Achenbach CJ, Kone B, et al. Clinical risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in Mali. Int J Infect Dis. 2019;81:149–55.
Lyu M, Wang D, Zhao J, et al. A novel risk factor for predicting anti-tuberculosis drug resistance in patients with tuberculosis complicated with type 2 diabetes mellitus. Int J Infect Dis. 2020;97:69–77.
Loewen PC, de Silva PM, Donald LJ, et al. KatG-mediated oxidation leading to reduced susceptibility of bacteria to kanamycin. ACS Omega. 2018;3(4):4213–9.
Acknowledgements
We appreciate from Infectious diseases department, The First Affiliated Hospital, Henan University of Science and Technology.
Funding
We have not received any funding for this research.
Author information
Authors and Affiliations
Contributions
LSP and HXJ contributed to design this study. LSP and LYL contributed to collect data. LSP analyzed the results and wrote the manuscript. HXJ and LSP provided overall supervision and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiment was approved by the Ethics Committee of the First Affiliated Hospital of Henan University of Science and Technology. This study used routinely collected data extracted anonymously from patient charts and was granted a waiver from obtaining informed consent by the Ethics Committee of the First Affiliated Hospital of Henan University of Science and Technology and the research content and process of this project comply with the international and national ethical requirements for biomedical research, and meet the medical ethical requirements stipulated in the preliminary rules of ethical review measures for biomedical research involving human.
Consent for publication
Not applicable.
Competing interests
There is no any conflict of interest among the all authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, S., Liang, Y. & Hu, X. Risk factors for multidrug resistance in tuberculosis patients with diabetes mellitus. BMC Infect Dis 22, 835 (2022). https://doi.org/10.1186/s12879-022-07831-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07831-3