Abstract
Background
Neisseria gonorrhoeae, the causative agent for sexually transmitted infection (STI) gonorrhoea, has emerged with a significant public health impact on acquiring resistance to antimicrobials available for treatment. The resistance of N. gonorrhoeae limit treatment options and contributed to high morbidity associated with gonorrhoea. Data on antimicrobial resistance (AMR) profiles in N. gonorrhoeae is scares in Zambia. This study aimed to determine the antibiotic susceptibilities in N. gonorrhoeae isolates from Lusaka, Zambia.
Methods
A prospective cross-sectional study was conducted on 630 STI patients who presented with urethral or vaginal discharge from 2019 to 2020. Urethral and endocervical secretions were cultured on Modified Thayer Martin agar and incubated at 36 °C ± 1 °C in 5% CO2 for 24 h. Identification of N. gonorrhoeae isolates was achieved by Gram stain, oxidase, nitrocefin disk, BactiCard Neisseria, and Viteck® Compact. The AMR profiles were determined using E-test. Statistical significant was determined by Pearson’s Chi-square test, Mann-Whitney U test, or logistic regression with p-values of < 0.05 indicating significance.
Results
A total of 630 patients were recruited of which 46% (290/630) with the median of 29 years and interquartile range (IQR) of 19–39 years were male. The median of the females was 26 years and IQR of 15–37 years. Neisseria gonorrhoeae was isolated from 19.4% (122/630) patients of which 72.9% (89/122) were male, with highest prevalence of isolation in the age category of 25–34 years. The prevalence of resistance was high to penicillin (85.2%), tetracycline (68.9%) and ciprofloxacin (59.8%) with MIC90 of 32 µg/mL, 8 µg/mL, and 8 µg/mL respectively. The isolates had reduced susceptibility to cefixime (1.6%), spectinomycin (4.9%) and (4.9%) for azithromycin. All isolates were susceptible to ceftriaxone. Risk factors associated with AMR were douching in females (AOR 6.69, 95% CI; 1.11–40.31, p = 0.039), female gender (AOR 7.64, 95% CI; 1.11–52.33, p = 0.048), HIV-positivity (AOR 26.59, 95% CI; 3.67–192.7, p = 0.005), no condom use or unprotected sex (AOR 5.48, 95% CI; 1.17–22.75 p = 0.026), sex trading (AOR 4.19, 95% CI; 1.55–11.33, p = 0.010), and over-counter treatment of ciprofloxacin (AOR 3.44, 95% CI; 1.17–22.75, p = 0.023).
Conclusion
The N. gonorrhoeae resistance to penicillin, tetracycline and ciprofloxacin was high necessitating revision of the treatment guidelines. However, no resistance to ceftriaxone was detected. Therefore, monitoring of antibiotic resistance remains critical in Zambia.
Similar content being viewed by others
Background
Gonorrhoea is a sexually transmitted infection (STI) caused by the bacterium Neisseria gonorrhoeae, which remains a major global public health concern because of its capacity to acquire high levels of resistance to antimicrobial agents available for treatment [1, 2]. In the recent decades, Neisseria gonorrhoeae has dramatically developed plasmid mediated and/or chromosomally mediated antimicrobial resistance (AMR) leading to the removal of the antimicrobial agent usages as standard first line treatment regimens [3]. The emerging resistance of the superbug to recommended dual therapy of third generation cephalosporins (3GCs) such as ceftriaxone, and macrolide azithromycin threatens to undermine preventive and control measures of gonorrheoa worldwide [4,5,6].
In 2020, the World Health Organization (WHO) estimated 82.4 million incident global cases of gonorrheoa, and second commonly sexually transmitted disease after Chlamydia trachomatis infections among adults of 15–49 years of age [2, 7, 8]. The highest incidence rate of gonorrhoea was found in the African region with 50–100 estimated new infections per 1,000 women and men respectively, every year [9]. In the absence of gonococcal vaccine, novel antimicrobial agents that are effective, affordable and accessible are vital to reduce substantial morbidity and spread of the superbug in the era of untreatable gonorrhoea [10,11,12].
Neisseria gonorrhoeae infects the genital tract in women and men and can also be transmitted from mother to child during delivery and cause infection of the eye of the newborn [13,14,15,16]. Complications of cervical gonorrhoea include pelvic inflammatory disease (PID), ectopic pregnancy, infertility, premature rapture of membranes (PROM), preterm birth, low birth weight, spontaneous abortions and neonatal ophthalmia which can progress to blindness in untreated cases of gonorrhoea [8, 17,18,19,20,21,22,23,24,25,26,27,28]. The dissemination of N. gonorrhoeae to extra-genital sites causes endocarditis, septic arthritis and meningitis [29, 30]. In addition to causing complications, gonorrhoea is highly associated with HIV acquisition and transmission by increasing the entry of infective inoculums in co-infections [27, 28, 31,32,33,34,35].
The syndromic case management of sexually transmitted infections (STIs) which had contributed highly to AMR due to empirical treatment was being used in many countries in sub-Sahara Africa [9, 36, 37]. The Zambian standard treatment guidelines recommended the use of single dose of ciprofloxacin in the treatment of gonorrhoea (Ciprofloxacin 500 mg PO stat Plus Doxycycline 100 bd PO X 7/7) [38]. There was scarce information on antimicrobial trends monitoring in N. gonorrhoeae for proper selection and use of antimicrobial agents despite resistance been an emerging phenomenon in Zambia. Multidrug-resistant (MDR) N. gonorrhoeae was defined as isolates resistant to either 3GCs or azithromycin, plus at least two antimicrobial agents (penicillin, ciprofloxacin, tetracycline, spectinomycin), and extensively drug-resistant (XDR) N. gonorrhoeae been resistant to 3GCs and azithromycin, plus at least two antimicrobial agents (penicillin, ciprofloxacin, tetracycline) [39].
The aim of this study was to determine the antimicrobial resistance profile of Neisseria gonorrhoeae isolated from patients attending sexually transmitted infection clinics in urban hospitals, Lusaka, Zambia.
Methods
Study design and population
A prospective cross-sectional study on 122 Neisseria gonorrhoeae isolated from 630 urogenital specimens from patients attending STIs clinics in urban hospitals in Lusaka, Zambia. The urethral and endocervical specimens were collected from patients who presented with a discharge from September, 2019 to August, 2020, and submitted for testing to microbiology laboratory at the University Teaching Hospital. The UTH microbiology laboratory participates in WHO and Southern Africa Development Accreditation Service (SADCAS) programs.
Data collection
The data was collected by trained nurses, clinical officers and medical doctors through face to face interviews technique using structured questionnaires to collect demographical and clinical factors from consented study participants. The questionnaires were adopted after reviewing different research studies done so far in the region.
Specimen collection
Urethral swabs from male participates were collected by inserted a flexible wire 2–3 cm into the urethra and rotated gently before withdrawing. In female participants the speculum was inserted to visualise the cervix and a Dacron swab on a rigid shaft was inserted 2–3 cm into the endocervix and removed with rotation from the endocervical canal. The swabs were immediately put in Amies transport media and transported to UTH microbiology laboratory for testing within 15 min of collection.
Bacterial culture and identification
Urethral and endocervical secretions were inoculated on Modified Thayer Martin medium and incubated at 36 ºC ± 1 °C in 5% CO2 for 24 h, and then the plate was read for growth. Identification of the isolates was achieved by Gram stain (GCC Diagnostics, Flintshire. UK. Lot: 1801), oxidase strips (SIGMA-ALDRICH Co., St. Louis, USA. Lot: BCCD8022), BactiCard Neisseria kit (REMEL Inc., Lenexa, KS 66,615 USA. Ref. R21110), and Vitek® Compact using NH ID cards (bioMerieux, Marcy-I’Etoile, France) according to the manufacturer’s protocol. The β-lactamase disk (Mast Group Ltd. Merseyside. UK. Ref. D59) was used to identify penicillinase-producing Neisseria gonorrhoeae strains (PPNG), and isolates were regarded as having a high level of resistance to tetracycline (TRNG) with MIC values ≥ 16 µg/mL.
Antimicrobial susceptibility testing
The minimum inhibitory concentrations (MICs; µg/mL) of ciprofloxacin, ceftriaxone, spectinomycin, azithromycin, penicillin, and tetracycline were determined by E-test (bioMerieux, Marcy-I’Etoile, France), on GC-chocolate with 1% Vitox supplement (Beckton Dickison, France) following the manufacturer’s instructions. The interpretation of MIC dilutions in susceptible (S), intermediate (I) and resistance (R) categories were according to Clinical and Laboratory Standard Institute (CLSI) criteria [40]. The plates were inoculated by dipping a sterile swab into a bacterial cell suspension adjusted to 0.5 McFarland standards using a turbidometer (Oxoid Integrated Technologies Ltd, England). The standardised inoculum was then streaked across the surface of the GC-chocolate agar. The plates were dried at ambient temperature for 5 min before applying the E-test strips and incubated at 36oC ± 1o C in 5% CO2 for 24 h. The SIR categories for antimicrobial agents in µg/mL were as follows: Ciprofloxacin (CIP) S; ≤0.06, I; 0.12–0.5, R; ≥1, ceftriaxone (CTX) S; ≤ 0.25, R; > 0.25, spectinomycin (SPEC) S; ≤ 32, I; 64, R ≥ 128, cefixime (CFX) S; ≤0.25, R; >0.25, azithromycin (AZT) S; ≤1, R; >1, penicillin (PEN) S; ≤0.06, I; 0.12-1, R; ≥2, and tetracycline (TET) S; ≤0.25, I; 0.12-1, R; ≥2. Neisseria gonorrhoeae American Type Culture Collection (ATCC) 49,226 was used as a reference strain and was within the acceptable quality control ranges.
Statistical analysis
Raw data was cleaned, audited and exported to Stata statistical software version 12.1 (Stata, California, USA) for analysis. Descriptive statistics was reported as frequencies and proportions. The Pearson’s Chi-square test was used to determine the bivariate association between individual categorical variables and the outcome variable. Mann-Whitney U test was used to determine the significant difference in the median age of study participants, and median and interquartile ranges were computed. Univariate and multiple logistic regression was used to assess the association between outcome variable and independent variables; the odds ratio (OR), p-values and 95% confidence interval were computed. A p-value of < 0.05 was taken as indication of statistical significance.
Results
A total of 630 participants were recruited of which 54% (340/630) were female. Neisseria gonorrhoeae was isolated from 19.4% (122/630) of which 72.9% (89/122) were from male. The gonococcal (GC) infection was much higher in males accounting for (73%), and (54.9%) of the participants with GC infection were married. The highest prevalence of GC infection (47.5%) occurred in the age group of 25–34 years, participants without tertiary education (73.7%), employees (68.9%), men who were circumcised (53.9%), and among men (50.8%) of who used condom during sexual intercourse. The HIV positivity among the participants was (26.2%), traded sex (48.8%), over counter treatment (27%) and (36.4%) of females participants were douching. The majority of GC infection (36.9%) were reported from University Teaching Hospital which was the largest and main referral medical centre in Zambia (Table 1).
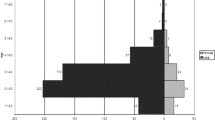
The median of age for males was 29 years (interquartile range [IQR], 19 to 39 years), and the median for females was 26 years (IQR, 15–37 years). There was no significant difference in median age between males and females across the categories of gender, p = 0.092 (Fig. 1).
Table 2 shows the antimicrobial profile of the 122 N. gonorrhoeae isolates, and 85.2% (104/122) isolates were resistant to penicillin with MIC values ranging from 0.047 to 32 µg/mL, MIC50 of 32 µg/mL and MIC90 of 32 µg/mL, while 68.9% (84/122) isolates were resistant to tetracycline with MIC range of 0.032 to 64 µg/mL, MIC50 of 4 µg/mL and MIC90 of 8 µg/mL. For ciprofloxacin, 59.8% (73/122) isolates were resistant with MIC values ranging from 0.016 to 32 µg/mL, MIC50 of 2 µg/mL and MIC90 of 8 µg/mL. Only 1.6% (2/122) isolates were resistant to cefixime with MIC range of 0.016 to 1 µg/mL, MIC50 of 0.032 µg/mL and MIC90 of 0.19 µg/mL. The resistance of isolates to azithromycin was 4.9% (6/122) with MIC ranging from 0.016 to 4 µg/mL, MIC50 of 0.125 µg/mL, and MIC90 of 0.75 µg/mL. All isolates were susceptible to ceftriaxone. The PPNG was detected in 45.1%, and TRNG in 2.5%, whereas 1.6% of the isolates presented both phenotypes (PPNG/TRNG).
Overall, 40.9% (50/122) isolates were resistant to three or more of the tested antimicrobial agents of which 4.9% (6/122) were MDR. Out of six MDR isolates, five were associated with azithromycin resistance and one isolate was associated with cefixime resistance. Only 5.7% (7/122) isolates were resistant to four antimicrobial .agents of which one isolate was XDR showing resistance to cefixime, azithromycin, ciprofloxacin and tetracycline (Fig. 2). The risk factors associated with N. gonorrhoeae resistance to ciprofloxacin, tetracycline and penicillin were gender; female (AOR 7.64, 95% CI; 1.11–52.33, p = 0.048), HIV-positivity (AOR 26.59, 95% CI; 3.67–192.7, p = 0.005), no condom use or unprotected sex (AOR 5.48, 95% CI; 1.17–22.75, p = 0.026), sex trading (AOR 4.19, 95% CI; 1.55–11.33, p = 0.010) over-counter treatment of ciprofloxacin (AOR 3.44, 95% CI; 1.17–22.75, p = 0.023) and douching in females (AOR 6.69, 95% CI; 1.11–40.31, p = 0.039) (Additional files 1, 2, 3: Tables S1, S2 and S3)
Discussion
Gonococcal infections have been on the increase and could be attributed to different factors in different regions [41]. Data presented in this study showed the highest isolation rate of gonorrhoea in male than female in young adults, and corroborates with findings in South Africa [42]. Neisseria gonorrhoeae circulating in Lusaka, Zambia are highly resistant to ciprofloxacin, penicillin and no isolate was resistant to ceftriaxone. The resistant N. gonorrhoeae was seven times more likely been isolated from older than younger patients. This could be attributed to vulnerability of the elderly to infections due to weaker immune systems subsequently intensive use of treatment drugs. The female patients are more associated with resistance than men, and those female patients who douche are six times more likely to suffer from AMR than those who were not practicing douching. Statistical association was found between douching and adverse health outcome among Zambian women [43]. HIV positivity, no condom use, and over-counter treatment are others risk factors that showed association with resistant strains of N. gonorrhoeae. These results are concordant with findings of the study done in Zimbabwe where HIV positivity, unprotected sex, and self-treatment were associated with high prevalence and resistance of N. gonorrhoeae among sexually active age groups [44].
The MDR and XDR N. gonorrhoeae showed higher levels of resistance in ciprofloxacin, penicillin and tetracycline as compared to non MDR and XDR N. gonorrhoeae. The superbugs showed resistance to cefixime and no resistance to ceftriaxone. N. gonorrhoeae strains were highly resistant to penicillin, tetracycline and ciprofloxacin. This is consistent with studies conducted in Europe, and East Asia where resistance to previously recommended treatment such as ciprofloxacin was generally above 50% [10]. According to WHO treatment guidelines the drug of resistance ≥ 5% threshold should not be recommended for treatment [45]. The findings of MDR and XDR pose serious clinical challenges as they indicate the emergence superbugs circulating in the hospitals in Lusaka, Zambia.
Conclusion
This study showed high prevalence of AMR of N. gonorrhoeae associated with various demographics and clinical variables. The N. gonorrhoeae showed high resistance to ciprofloxacin, penicillin and tetracycline which poses a treatment challenge. The high level of resistance in ciprofloxacin and tetracycline highlights the need for revision of the national treatment guidelines, and monitoring of antibiotic resistance remains critical in Zambia.
Limitation of the study
The isolates studied were only from three hospitals in Lusaka and might not be representative of other settings in Zambia.
Availability of data and materials
All data generated during the current study are included in this published article and its Additional files.
References
Unemo M, Lahra MM, Cole M, Mike S, Galarza P, Ndowa F, Martin I, Dillion JR, Ramon-Pardo R, Bolan G, Wi T. World Health Organisation Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. J PubMed Central. 2019;16:412–25.
WHO. Global progress report on HIV, viral hepatitis and sexually transmitted infections, May 20, 2021. https://www.who.int/publications/i/item/9789240027077. Accessed Aug 18, 2021.
Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st Century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613.
World Health Organisation (WHO). WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva: WHO; 2016.
Unemo M, Ross J, Serwin AB, Gomberg M, Cusini M, Jensen JS, Unemo M, Ross J, Serwin AB, Gomberg M, Cusini M, Jensen JS. European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J Sexually Transm Dis Acq Immunodef Dis Syndr. 2020;2020:956462420949126.
Fifer H, Saunders J, Soni S, Sadiq ST, FitzGerald M. UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J Sexually Transm Dis Acq Immunodef Dis Syndr. 2020;31:4–15.
World Health Organisation (WHO). Report on global sexually transmitted infection surveillance, 2018. Geneva: WHO; 2018. 'http://www.who.int./reproductivehealth/publications/rtis/gonorrhoeae-treatment-guidelines/en/.
Unemo M, Seifert HS, Hook EW, Hawkes S, Ndowa F, Dillion JR. Gonorrhoea. Nat Rev Dis Primers. 2019;5:97.
Lowe S, Mudzviti T, Mandiriri A, Shamu T, Mudhokwani P, Chimbetete C, et al. Sexually transmitted infections, the silent partner in HIV-infected women in Zimbabwe. South Afr J HIV Med. 2019;20(1):849.
Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 2017;14:e1002344.
Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613.
Alirol E, Wi TE, Bala M, Bazzo ML, Chen XS, Deal C, Dillon JR, Kularatne R, Heim J, van HooftHuijsduijnen R, Hook EW, Lahra MM, Lewis DA, Ndowa F, Shafer WM, Tayler L, Workowski K, Unemo M, Balasegaram M. Multidrug-resistant gonorrhoea: a research and development roadmap to discover new medicines. PLoS Med. 2017;14:e1002366.
Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, Marrazzo JM, Sonder GJB, Schwebke JR, Hoornenborg E, Peeling RW, Philip SS, Low N, Fairley CK. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17:e235–79.
Namraj Goire MML, Chen M, Donovan B, Christopher K, Fairley RG, Kaldor J, Regan D, Ward J, Nissen MD, Sloots TP, Whiley DM. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol. 2014;12:223–9.
Amanda B. Gonorrhea. 2008. http://www.austincc.edu/microbio/2704w/ng. Accessed 22 Mar 2018.
WHO. Sexual and reproductive health: WHO; 2017. http://www.who.int/reproductivehealth/topics/rtis/amr-gonorrhoea-on-the-rise/en/. Accessed 22 Mar 2018.
Liu B, Roberts CL, Clarke M, Jorm L, Hunt J, Ward J. Chlamydia and gonorrhoea infections and the risk of adverse obstetric outcomes: a retrospective cohort study. Sex Transm Infect. 2013;89(8):672–8.
Donders GG, Desmyter J, De Wet DH, Van Assche FA. The association of gonorrhoea and syphilis with premature birth and low birth weight. Genitourin Med. 1993;69(2):98.
Maxwell GL, Watson WJ. Preterm premature rupture of membranes: results of expectant management in patients with cervical cultures positive for group B streptococcus or Neisseria gonorrhoeae. Am J Obstet Gynecol. 1992;166(3):945–9.
Pathela P, Braunstein SL, Blank S, Schillinger JA. HIV incidence among men with and those without sexually transmitted rectal infections: estimates from matching against an HIV case registry. Clin Infect Dis. 2013;57(8):1203–9.
Kirkcaldy RD, Weston E, Segurado AC, Hughes D. Epidemiology of gonorrhoea: a global perspective. J Sex Health. 2019;16:401–11.
Elias JF, Vogel U. Manual of clinical microbiology. In: Carroll CC et al. (eds) 12th edn. vol. 1. American Society for Microbiology. 2019. pp. 640–655.
Hook EW 3rd, Handsfield HH. Sexually transmitted diseases. In: Holmes KK et al. (eds) This comprehensive chapter describes different clinical manifestations of gonorrhoea. McGraw- Hill Education 2008; 4th pp. 627–645
Public Health Agency of Canada. Canadian Guidelines on Sexually Transmitted Infections - Management and treatment of specific infections - Gonococcal Infections (Government of Canada, Ottawa, 2013) (modified Sept 2017).
Costa-Lourenço APR, Su X, Le W, Yang Z, Patts GJ, Massari P, Genco CA. Epidemiological and clinical observations of gonococcal infections in women and prevention strategies. Vaccines. 2021;9:327.
Lovett A, Duncan JA. Human immune responses and the natural history of Neisseria gonorrhoeae infection. Front Immunol. 2019;9:3187.
Stevens JS, Criss AK. Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: neutrophilic host response, sustained infection, and clinical sequelae. Curr Opin Hematol. 2018;25:13–21.
Goire N, Lahra MM, Chen M, et al. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol. 2014;12:223–9.
Ali S, Sewunet T, Sahlemariam Z, Kibru G. Neisseria gonorrhoeae among suspects of sexually transmitted infection in Gambella hospital, Ethiopia: risk factors and drug resistance. BMC Res Notes. 2016;9(1):439.
Fingerhuth SM, Bonhoeffer S, Low N, Althaus CL. Antibiotic-resistant Neisseria gonorrhoeae spread faster with more treatment. Not More Sexual Partners PLoS Pathog. 2016;12(5):e1005611.
World Health Organization. Global action plan to control the spread and impact of antimicrobial resistance. In: Neisseria gonorrhoeae. Geneva: World Health Organization; 2012. 97892 41503 501/ en/.
Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17.
Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhoea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53(4):537–43.
Sadiq ST, Taylor S, Copas AJ, Bennett J, Kaye S, Drake SM, et al. The effects of urethritis on seminal plasma HIV-1 RNA loads in homosexual men not receiving antiretroviral therapy. Sex Transm Infect. 2005;81(2):120–3.
Cohen MS, Council OD, Chen JS. Sexually transmitted infections and HIV in the era of antiretroviral treatment and prevention: the biologic basis for epidemiologic synergy. J Int AIDS Soc. 2019;22(Suppl 6):e25355.
National Department of Health Republic of South Africa. First line comprehensive management and control of sexually transmitted infections (STIs). Protocol for the management of a person with a sexually transmitted infection. https://www.fidssa.co.za/Guidelines. 2010.
Verma R, Sood S. Gonorrhoea diagnostics: an update. Indian J Med Microbiol. 2016;34(2):139.
Ministry of Health, Zambia National Formulary Committee. Standard Treatment Guidelines, Essential Medicines List, Essential Laboratory Supplies for Zambia, 5th edition. Zambia Ministry of Health, Lusaka. 2020. https://www.moh.gov.zm
Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2009;7(7):821–34.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI-M100. 31st ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2021.
World Health Organization (WHO). Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. Geneva: WHO; 2012. https://apps.who.int/iris/handle/10665/44863. Accessed 19 July 2020.
Rambaran S, Naidoo K, Dookie N, Moodley P, Sturn WA. Resistance profile of Neisseria gonorrhoeae in KwaZulu, South Africa questioning the effect of the currently advocated dual therapy. J Sex Transm Dis. 2019;46(4):266–70.
Hamoonga TE, Olowski P, Musonda P. Vaginal douching in Zambia: a risk or benefit in women in the fight against cervical cancer: a retrospective cohort study. BMC Women’s Health. 2019;19:135.
Martin K, Olaru DI, Buwu N, Bandason T, Marks M, Dauya E, Muzangwa J, et al. Uptake of and factors associated with testing for sexually transmitted infections in community-based settings among youth in Zimbabwe: a mixed-methods study. Lancet Child Adolesc Health. 2021;5:122–32.
Martin K, Olaru DI, Buwu N, Bandason T, Marks M, Dauya E, et al. Uptake of and factors associated with testing for sexually transmitted infections in community-based settings among youth in Zimbabwe: a mixed method study-methods study. Lancet Child Adolesc Health 2021; 122–32.
Acknowledgements
We are very grateful to all staff members at Dermato-venereology department, Microbiology laboratory at the University Teaching Hospital, and Tropical Gastroenterology and Nutrition, School of Medicine, University of Zambia.
Funding
This work was supported by the Ministry of Health, Department of Biomedical Sciences, University of Zambia, Department of Dermato-venereology, University Teaching Hospital, Department of Pathology and Microbiology, University Teaching Hospital.
Author information
Authors and Affiliations
Contributions
KLS and GK designed the study and performed the laboratory work. AS and KLS were involved in data analysis. ON, MCM, SMM and KLS wrote the first draft of the manuscript. All authors read, edited and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All examined gonococcal isolates were cultured and preserved as part of the routine diagnostics, and information obtained during the study was kept confidential as no patient identification information was available in the study. Informed consent was obtained for all participants in the study. The study including its protocol was approved by Zambia National Health Research Ethics Board (ZNHREB Ref No.E17020), and the University of Zambia Health Sciences Research Ethics Committee (UNZAHREC Ref No. 20190624004). The whole study was carried out in accordance with STI guidelines and regulations at Dermato-venerology Unit at University Teaching Hospital, Lusaka, Zambia, which are in agreement with Declaration with Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: TableS1.
Association of demographics and clinicalvariables with N. gonorrhoeaeresistance to ciprofloxacin.
Additional file 2: TableS2.
Association of demographics and clinicalvariables with N. gonorrhoeaeresistance to tetracycline.
Additional file 3: TableS3.
Association of demographics and clinicalvariables with N. gonorrhoeaeresistance to penicillin.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sarenje, K.L., Ngalamika, O., Maimbolwa, M.C. et al. Antimicrobial resistance of Neisseria gonorrhoeae isolated from patients attending sexually transmitted infection clinics in Urban Hospitals, Lusaka, Zambia. BMC Infect Dis 22, 688 (2022). https://doi.org/10.1186/s12879-022-07674-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07674-y