Abstract
Background
This study was designed to evaluate the prevalence, genotypic patterns, and predominant mutations of hepatitis B virus (HBV) infection among diabetic patients.
Methods
Serum samples were obtained from 733 patients with type 2 diabetes mellitus and 782 non-diabetic controls. The presence of HBsAg and HBcAb was determined by ELISA. Nested PCR, targeting S and pre-core regions of the HBV genome, followed by sequencing was carried out to determine HBV genotypes and predominant mutations in the S, basal core promoter (BCP), and pre-core regions of the HBV genome.
Results
Of 733 diabetic patients, 94 cases (12.82%) were positive for HBcAb, 28 cases (3.82%) were positive for HBsAg, and 19 cases (2.59%) had HBV-DNA with genotype D, sub-genotype D1/D3 and subtype ayw2. An occult HBV infection was found in one of the HBV DNA-positive samples, which was positive for HBcAb but negative for HBsAg. P120T/G145R, G1896A/G1899A, and A1762T/G1764T were the most frequent point substitution mutations detected in the S, pre-core, and BCP regions of the HBV genome, respectively. P120T and G145R mutations were associated with low levels or undetectable levels of HBsAg in serum. Therefore, routine tests based on HBsAg detection cannot detect HBsAg-negative infected patients.
Conclusions
Relatively high prevalence of HBV infection was found in diabetic patients, while all of the HBV-infected patients were unaware of their infection. Therefore, screening for HBV infection should be included in the management program of diabetes for timely diagnosis and treatment of infected but asymptomatic patients.
Similar content being viewed by others
Introduction
Hepatitis B virus (HBV) infection, with approximately 296,000,000 chronic infections globally and an estimated 820,000 deaths annually, is considered to be a challenging public health problem [1]. HBV is a small virus in the family Hepadnaviridae, with a partially double-stranded circular DNA genome and enveloped icosahedral capsid. The genome contains four overlapping open reading frames called S, pre-C/C, P, and X ORFs, which encode surface proteins (HBsAg), e antigen (HBeAg), and core protein (HBcAg), polymerase, and X protein [2, 3].
The lack of proofreading activity in the replication process of HBV results in the emergence of a high rate of mutations in the HBV genome [4]. The most prevalent mutations that occur naturally during HBV infection are pre-core (pre-C) and basal core promoter (BCP) mutations [5, 6]. The pre-C and BCP mutations favor the persistence of HBV infection and subsequently increase the risk of liver cirrhosis and hepatocellular carcinoma (HCC) [4]. Besides, some mutations in the S region are responsible for the weak response to immunotherapy and HBV vaccination as well as the failure of HBsAg detection [7,8,9]. Therefore, detection of these mutations can be beneficial in predicting response to HBV vaccination and antiviral treatment as well as the progress of HBV infection in high-risk groups.
Diabetes mellitus, a group of metabolic disorders characterized by hyperglycemia, is recognized as one of the most prevalent non-communicable diseases around the world [10]. There are currently about 422 million diabetic patients and 1.5 million death-related diabetes in the world [11]. Although the cause of diabetes mellitus is not fully understood, several known risk factors, including obesity, aging, chronic inflammation, genetic predisposition, physical inactivity, and urbanization, are thought to promote the development of diabetes mellitus [12]. In addition to the role of genetics, biology and demographic factors, recent studies have highlighted the link between HBV infection and the development of diabetes mellitus [13,14,15,16]. The coexistence of HBV infection and diabetes mellitus exhibits a life-threatening condition, which requires immediate consideration. Although nearly 11.9% of the adult population in Iran are diabetics [17], the current knowledge on molecular evaluation of HBV infection among the diabetic population is scarce in Iran. Therefore, this study was designed to evaluate the prevalence, possible risk factors, genotypic patterns, and predominant mutations of HBV infection among patients with type 2 diabetes mellitus.
Subjects and methods
Patients and sample collection
All of the patients with type 2 diabetes mellitus attending diabetic clinics of the Bushehr University of Medical Sciences located in southern Iran were included consecutively in this study. In accordance with the American Diabetes Association criteria, diabetic cases were defined as patients with fasting serum glucose levels ≥ 126 mg/dL on at least 2 different occasions accompanied by regular use of oral antidiabetic medications [18]. The demographic, laboratory, and clinical data of each patient were obtained from the patient’s medical records at the diabetic clinics. As a control group, 782 non-diabetic volunteers, who were matched in sex, age (± 3 years), and date of participation with diabetic patients, were recruited from the out-patient populations attending the hospitals of the Bushehr University of Medical Sciences for blood tests. The patients were excluded if they had markers of HCV and/or HIV infections and exhibited evidence of hepatogenous diabetes, gestational diabetes, type 1 diabetes, and autoimmune or metabolic chronic liver disease. All of the participants were informed about the purpose of the research and requested to sign a written informed consent to use their leftover serum samples for HBV detection and analysis. The study was approved by the Ethical Committee of the Bushehr University of Medical Sciences with research project number IR.BPUMS.REC.1395.54 and was funded by the Deputy Research and Affairs of the University with grant number 3254. In addition, all methods were performed under the relevant guidelines and regulations.
Laboratory diagnosis
All of the serum samples were tested for the detection of hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (HBcAb) using HBsAg one-Version ULTRA and HBc Ab ELISA kits (DIA.PRO, Milan, Italy), respectively. The sensitivity and specificity of these kits were 100%. The HBsAg and/or HBcAb seropositive samples were tested using nested PCR, targeting the S region of the HBV genome, followed by sequencing to determine the genotypic pattern of HBV infection. Briefly, HBV DNA was extracted from serum samples using the High Pure Viral Nucleic Acid kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. To determine HBV genotypes, 447 nucleotides and 416 nucleotides of the S region were amplified by nested PCR using outer primers [forward primer (244-HBS-F1): GAGTCTAGACTCGTGGTGGACTTC; reverse primer (691-HBS-R1): AAATKGCACTAGTAAACTGAGCCA] and inner primers [forward primer (255-HBS-F2): CGTGGTGGACTTCTCTCAATTTTC; reverse primer (671-HBS-R2): GCCARGAGAAACGGRCTGAGGCCC], respectively [19,20,21].
To determine mutations in BCP and pre-C regions, the pre-C region was amplified in the first round of PCR using primers 1606-pre-C-F1 (GCATGGAGACCACCGTGAAC) and 2395-pre-C-R1 (AGGCGAGGGAGTTCTTCTTC). The second round of PCR was performed using primers 1653-pre-C-F2 (CATAAGAGGACTCTTGGACT) and 2393-pre-C-R2 (GCGAGGGAGTTCTTCTTC) [21,22,23]. The 789 bp and 740 bp length fragments from the pre-C region were amplified in the first and second rounds of nested PCR, respectively. The sequences of primers and PCR conditions for amplification of the S and pre-C regions of the HBV genome are summarized in Table 1.
Genotyping and mutations analyses
The PCR products were analyzed using agarose gel electrophoresis. Following the extraction of amplicons from the agarose gel using the Agarose Gel DNA Extraction Kit (Roche, Mannheim, Germany), the 416 bp length fragments from the S region and the 740 length fragments from the pre-C region were sequenced to determine HBV genotypes and mutations by Sanger dideoxy sequencing technology (Macrogen Co., Korea). The obtained sequences were analyzed by using GenBank Basic Local Alignment Search Tool (BLAST), compared with the reference sequences of S and pre-C regions of the standard HBV genotypes available at the nucleotide database of the NCBI and submitted to the GenBank sequence database. Then, the S and pre-C sequences isolated from the patients and the reference sequences were aligned by the ClustalW program in the Molecular Evolutionary Genetics Analysis (MEGA) software version 7.0 (Biodesign Institute, Tempe, AZ, USA) [24]. The phylogenetic tree was constructed by the neighbor-joining method using MEGA software, as described previously [25]. The reference strains representing the standard HBV genotypes were used as references.
Statistical analysis
The Student’s t-test was used to analyze and compare quantitative variables between HBV-positive and HBV-negative diabetic patients. Categorical variables were compared by χ2 test or Fisher’s exact test. Logistic regression analysis was used to determine the risk factors associated with the prevalence of HBV infection among diabetic patients, and the odds ratio with 95% confidence intervals was calculated. SPSS 17 package program (SPSS Inc., Chicago, IL, USA) was used for data analyses, and P values < 0.05 were considered significant.
Results
Baseline demographic
Serum samples were obtained from 733 patients with type 2 diabetes mellitus, including 256 males and 477 females. The mean age ± SD of diabetic patients was 55.2 ± 11.7 years with a range of 26–96 years. The majority of diabetic patients were in the age groups 51–60 years (38.7%) and 61–70 years (20.1%), respectively. Moreover, 782 non-diabetic volunteers, including 304 males and 478 females, were enrolled in the study as the control group. The mean age ± SD of the non-diabetic controls was 53.37 ± 13.36 years with a range of 20–93 years.
HBV sero-markers and risk factors
Of 733 patients with type 2 diabetes mellitus, 94 cases (12.82%, 95% CI 10.60–15.44%) had HBcAb, and 28 cases (3.82%, 95% CI 2.66–5.47%) were positive for HBsAg. The seroprevalence of HBsAg and HBcAb in the non-diabetic controls were 1.15% (95% CI 0.61–2.17%) and 10.74% (95% CI 8.76–13.11%), respectively. Diabetic patients had a significantly higher seroprevalence of HBsAg than the non-diabetic controls (P = 0.01). Although diabetic patients had a higher seroprevalence of HBcAb than the non-diabetic controls, the difference was not statistically significant (P = 0.23) (Additional file 1: Table S1).
The highest rate of HBV seroprevalence was observed in the age group 61–70 years (6.1%) for HBsAg and in the age group > 71 years (30.3%) for HBcAb, whereas the lowest HBcAb seroprevalence was found in the age group 26–30 years (6.25%), and those aged < 40 years did not show HBsAg seropositivity (Additional file 1: Tables S2 and S3). Overall, HBV seropositivity increased with age so that HBsAg seropositive diabetic patients had a significantly higher mean age (59.92 ± 13.2) compared to HBsAg seronegative diabetic patients (55.00 ± 11.66) (P = 0.03). Besides, HBcAb seropositive diabetic patients showed significantly higher mean age (60.31 ± 12.57) compared to HBcAb seronegative diabetic patients (54.44 ± 11.44) (P = 0.001). HBsAg and HBcAb seropositivity were more prevalent in female diabetic patients than male patients, although the differences were not statistically significant (Additional file 1: Tables S2 and S3). HBsAg seropositive diabetic patients had higher AST and ALT levels but lower TCH, TG, and FBS levels than HBsAg seronegative patients. Nevertheless, the seroprevalence of HBsAg among diabetic patients was not statistically associated with the serum levels of liver enzymes, TCH, TG, and FBS (Table 2).
HBsAg seroprevalence was higher among the non-diabetic controls aged 70 to 79 years (4.5%), whereas those aged 50–59 years had the lowest rate of HBsAg seroprevalence (0.7%) (Additional file 1: Table S4). HBcAb seroprevalence increased with age so that the non-diabetics over 80 years old had a significantly higher HBcAb seroprevalence (36.7%) compared to the other age groups (P = 0.0001) (Additional file 1: Table S5). Besides, HBsAg and HBcAb seroprevalence was more prevalent in male non-diabetics than female non-diabetics, although the differences were not statistically significant (Additional file 1: Tables S4 and S5).
Molecular evaluation and genotyping
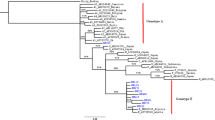
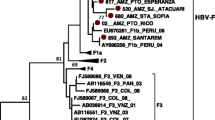
According to the molecular evaluation, 19 diabetic patients (2.59%, 95% CI 1.67–4.01%) had HBV-DNA (Additional file 1: Figs. S1 and S2). The mean age of HBV DNA-positive diabetic patients (60.0 ± 14.3) was higher than that of HBV DNA-negative diabetic patients (55.07 ± 11.66), but this difference was statistically insignificant (P = 0.07). Overall, HBV-DNA positivity in diabetic patients was not significantly associated with age, gender distribution, and the serum levels of AST, TCH, TG, and FBS (Table 3). In contrast, HBV-DNA positivity was associated with ALT levels so that those patients with ALT levels of ≥ 81 IU/L (13.0%) had a significantly higher prevalence of HBV-DNA positivity than those patients with ALT levels of ≤ 24 IU/L (1.9%) (OR: 9.60; 95% CI 1.51–61.07; P = 0.02). Therefore, an ALT level of ≥ 81 IU/L was the only significant predictive variable for HBV-PCR positivity in diabetic patients (Table 3). An occult HBV infection (OBI) was found in one of the HBV-DNA positive samples, which was positive for HBcAb but negative for HBsAg. The prevalence of HBV-DNA positivity among diabetic patients (2.59%) was significantly higher than HBV-PCR positivity among the non-diabetic controls (0.77%, 95% CI 0.36–1.66%) (P = 0.007). None of the non-diabetic controls had occult HBV infection. The HBV sequences isolated from patients with type 2 diabetes mellitus were identified as genotype D, sub-genotype D1/D3, and subtype ayw2 (Fig. 1). The same genotypic pattern was found among the non-diabetic controls. Subtype ayw2 was characterized by Arg (R) at positions 122, Pro (P) at position 127, and Lys (K) at position 160 of HBsAg, but not Phe (F) and Ala (A) at positions 134 and 159 of HBsAg, respectively (Fig. S3). The HBV sequence isolated from diabetic patient with OBI was genotype D, sub-genotype D1/D3, and subtype ayw2.
Neighbor-joining phylogenetic tree based on ~ 380 bp nucleotide sequence (~ 270 to 670 bp of the complete reference genome) of the S region of HBV isolates from the serum samples of patients with type 2 diabetes mellitus (green squares) (MF419214–MF419229) and non-diabetic controls (blue triangles) (NDC1–NDC3). Bootstrap resampling strategy and reconstruction were carried out 1000 times to confirm the reliability of the phylogenetic tree
Mutation analysis
Frequent point substitution mutations were detected in the S, pre-C, and BCP regions of the HBV genome isolated from the patients. Of those mutations in the S region, the conversion of Pro → Thr at amino acid 120 (P120T) of HBsAg was detected in five samples with HBsAg OD near the cut off of the ELISA kit, and conversion of Gly → Arg at amino acid 145 (G145R) of HBsAg was detected in one sample (Table 4 and Additional file 1: Fig. S3). The S G145R/P120T double mutation was observed in one HBsAg-negative sample. This sample was an OBI case (Accession No. MF419215). In the samples with P120T and/or G145R mutations, HBV-DNA was detectable in the second round of nested PCR. Of those mutations in the pre-C region, a G to A substitution at nucleotide position 1896 (G1896A) was detected in four samples, whereas G1899A mutation was found in eight samples. G1899A mutation was detected in the pre-C region of the occult HBV strain (Accession No. OK382075). Six samples had 5 nucleotide substitutions in the BCP region, including T1753C, A1762T, G1764T, C1766G, and C1773T. These 5 nucleotide substitutions were detected in the BCP region of the occult HBV strain (Accession No. OK382075). There were two samples with pre-C G1896A/G1899A double mutation, while six samples had BCP A1762T/G1764T double mutation. The serum samples with pre-C G1896A mutation exhibited no concomitant BCP A1762T and G1764T mutations; however, six out of the eight samples with pre-C G1899A mutation also exhibited A1762T and G1764T mutations (Table 5 and Additional file 1: Fig. S4). The A1762T and G1764T mutations in the BCP region caused the conversion of Lys → Ile at amino acid 130 (K130I) and Val → Leu at amino acid 131 (V131L) of the X protein (Table 5 and Additional file 1: Fig. S5). I127T, K130I, V131L were detected in the X protein of the occult HBV strain (Accession No. OK382075). The mutation analysis of the OBI case (patient no. 63) has been reported in tables 4 and 5. Moreover, the possible mutations in the S, pre-C, and BCP regions of the HBV genome are shown in Additional file 1: Table S6.
Discussion
In this study, 3.8% and 12.82% of diabetic patients were positive for HBsAg and HBcAb compared to 1.15% and 10.74% of the non-diabetic controls, and 2.59% of diabetic cases had HBV-DNA compared to 0.77% of the non-diabetic controls. Therefore, diabetic patients had a significantly higher prevalence of HBV infection than the controls. Besides, the HBsAg seroprevalence of 3.8% observed in diabetic patients is considerably higher than the HBsAg prevalence of 0.15% reported in the blood donors of this region [26]. Moreover, the HBV prevalence reported in this study is higher than the overall prevalence of HBV in the general population of Iran [27]. The high prevalence of HBV infection in diabetic cases might be due to the possible role of HBV in the development of diabetes mellitus through induction of insulin resistance associated with persistent inflammatory reactions in response to HBV infection and overproduction of tumor necrosis factor-α and nitric oxide in the liver which are involved in the destruction of the insulin metabolic action, destruction of β-cell of the islet due to the replication of HBV in the pancreas, or induction of glycometabolism disorders due to hepatic damage caused by HBV infection [14, 15]. On the other hand, the risk factors associated with diabetic patients, including frequent blood glucose monitoring, hospitalization, and medical interventions, increase the risk of exposure to HBV [16]. In addition to parenteral transmission of HBV, defective cellular immune responses due to altered levels of T-lymphocyte subsets increase the risk of infection in diabetic patients [16]. Furthermore, concurrent diabetes mellitus increases the risk of hepatocarcinogenesis in HBV-infected patients [28, 29].
The association between hepatitis B and diabetes mellitus remains controversial. Some studies demonstrated an association between HBV infection and diabetes mellitus [13,14,15,16], while no such association was reported in some other studies [10, 30]. These studies were performed in different geographical regions with different sample sizes and failed to reach a consensus. The current study indicates that diabetic patients have a higher risk of HBV infection when compared with non-diabetic subjects; however, given the fact that this is a cross-sectional study, prospective cohort studies are required to confirm this assertion and to achieve further understanding of the possible link between HBV infection and diabetes mellitus in an Iranian population.
The seroprevalence of 3.8% for HBsAg reported in this study is higher than those observed among diabetic patients in Italy (1.63%) [31], the Kurdistan Region of Iraq (2.13%) [32], and Ethiopia (3.7%) [33] but lower than those reported among diabetic patients in China (13.5%) [34] and Taiwan (13.54%) [35]. Moreover, the seroprevalence of 12.82% for HBcAb observed in this study is higher than that reported among diabetic patients in the United States (8.2%) [36] but lower than those reported among diabetic patients in Brazil (16.8%) [37] and China (62.3%) [34]. Differences in immunization status, risk factors, the burden of HBV infection in the general population, the levels of safety measures in public health centers, preventive strategies, and risk of exposure to HBV in different regions as well as differences in the sensitivity and specificity of diagnostic methods, study period, sociodemographic characteristics of the study population and number of participants in different studies might explain these variations in the seroprevalence of HBV infection in different parts of the world.
In the present study, HBsAg seropositivity increased with age, from 3% in the age group 41–60 years to 6.7% in the age group over 60 years, while all diabetic patients under 40 years old were negative for HBsAg. This is probably due to the implementation of the HBV vaccination program for infants in 1993 and the vaccination of teenagers since 2006 in Iran [26]. Our findings are in accordance with the results of a previous study among blood donors in the South of Iran, which has demonstrated a significant association between older ages and higher HBsAg seroprevalence [26]. Besides, all of the HBV-infected diabetic patients were unaware of their infection, although they had higher AST and ALT levels than uninfected diabetic patients. A study from Italy also reported higher levels of liver enzymes in infected diabetic patients compared to uninfected cases [31]. In the clinic, elevated liver enzymes are usually considered signs of metabolic disease, not signs of HBV infection.
In this study, P120T/G145R, G1896A/G1899A, and A1762T/G1764T were the most frequent point substitution mutations detected in the S, pre-core, and BCP regions of HBV isolated from the patients, respectively. P120T and G145R mutations in the S region were associated with low levels or undetectable levels of HBsAg in serum. Therefore, routine tests based on HBsAg detection cannot detect HBsAg-negative infected patients. Besides, HBV with P120T and G145R mutations is thought to be a vaccine-induced immune escape mutant [9], since the patient with these two mutations has been immunized with the HBV vaccine. A1762T and G1764T mutations in the BCP region were detected in six samples. A high prevalence of A1762T and G1764T mutations has been reported in patients with liver fibrosis, cirrhosis, and HCC [4, 38]. The G1896A and G1899A mutations in the pre-C region were detected in four and eight samples, respectively. The pre-C mutations have a significant association with remission of liver disease [5]. Considering the critical impact of these mutations on the clinical course and treatment of HBV infection, prompt detection of these mutants in patients with chronic HBV infection is important and can improve the diagnostics, vaccination, and treatment strategies. According to the current HBV treatment strategies, tenofovir and entecavir are used in the treatment of chronic hepatitis B in Iran [39]. Deletion, insertion, or frameshifting mutations were not observed in our isolates.
Based on the nucleotide sequence analysis of the S region, HBV genotype D with sub-genotype D1/D3, and subtype ayw2 were found in our HBV DNA-positive diabetic patients. The same genotypic pattern has been reported in a previous study from southern Iran [40]. Genotype D with nine sub-genotypes (D1–D9) shows a widespread distribution and is predominant in the Mediterranean region, Europe, Africa, India, and Indonesia [41]. This genotype is characterized by chronicity, progression to cirrhosis and HCC, mutation frequency, and a low response to interferon-based therapy [41, 42]. HBV has been classified into 4 major serotypes (ayr, adw, adr and ayw) and 10 related subtypes (ayr, adw2, adw3, adw4, adrq+, adrq−, ayw1, ayw2, ayw3 and ayw4) according to the variations in amino acids at positions 122 and 160 [43]. Moreover, the frequency of mutations has a significant association with the predominant genotypic pattern of HBV in distinct geographical locations. For example, a high frequency of A1762T and G1764T mutations in the BCP region are associated with HBV genotypes C and D [7]. Besides, the pre-C G1896A mutation is characteristic of HBV genotypes B, D, and E [5], and subtype ayw is associated with genotypes D and E [2].
This is the first report on the molecular evaluation and mutation analysis of HBV infection among diabetic patients in Iran. However, the cross-sectional design of the study prevents follow-up of the infected patients. Therefore, the possible effects of these mutations on the disease progression remained unclear among diabetic patients. As another limitation, this study was not able to determine the relationship between HBV infection and diabetes mellitus. Therefore, whether HBV infection triggers the onset of diabetes or diabetes increases the risk of HBV infection remains to be determined by prospective or longitudinal studies.
Conclusion
The present study indicates a relatively high prevalence of HBV infection among patients with type 2 diabetes mellitus, which may remain undiagnosed in the absence of testing. Considering the complications associated with the coexistence of hepatitis B and diabetes, vaccination and screening of all diabetic patients for HBV infection as well as timely treatment of infected patients are recommended to reduce the incidence and risk of HBV infection among the diabetic population. Moreover, we observed P120T and G145R mutations in the S region, which were associated with low levels or undetectable levels of HBsAg in serum or plasma. Therefore, routine tests based on HBsAg detection cannot detect HBsAg-negative infected patients. Besides, A1762T/G1764T mutations in the BCP region and G1896A/G1899A mutations in the pre-C region were detected in our HBV-infected diabetic patients. Detection of these mutations can provide information regarding disease progression in patients with chronic HBV infection.
Availability of data and materials
All relevant data are within the paper or supplementary material. The S, pre-core, and X sequences isolated from diabetic patients have been submitted to the GenBank sequence database under GenBank accession Nos. MF419214–MF419229, OK382075-OK382085 and OK382075-OK382085, respectively. https://www.ncbi.nlm.nih.gov/popset?DbFrom=nuccore&Cmd=Link&LinkName=nuccore_popset&IdsFromResult=1391900023.
References
World Health Organization. Hepatitis B. 2019. World Health Organization https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
Kramvis A. Genotypes and genetic variability of hepatitis B virus. Intervirology. 2014;57(3–4):141–50.
Rajput MK. Mutations and methods of analysis of mutations in Hepatitis B virus. AIMS Microbiol. 2020;6(4):401–21.
Yan L, Zhang H, Ma H, Liu D, Li W, Kang Y, et al. Deep sequencing of hepatitis B virus basal core promoter and precore mutants in HBeAg-positive chronic hepatitis B patients. Sci Rep. 2015;5:17950.
Juniastuti, Utsumi T, Aksono EB, Yano Y, Soetjipto, Hayashi Y, et al. Predominance of precore mutations and clinical significance of basal core promoter mutations in chronic hepatitis B virus infection in Indonesia. Biomed Rep. 2013;1(4):522–8.
Tacke F, Gehrke C, Luedde T, Heim A, Manns MP, Trautwein C. Basal core promoter and precore mutations in the hepatitis B virus genome enhance replication efficacy of Lamivudine-resistant mutants. J Virol. 2004;78(16):8524–35.
Lin CL, Kao JH. Natural history of acute and chronic hepatitis B: the role of HBV genotypes and mutants. Best Pract Res Clin Gastroenterol. 2017;31(3):249–55.
El Chaar M, Candotti D, Crowther RA, Allain JP. Impact of hepatitis B virus surface protein mutations on the diagnosis of occult hepatitis B virus infection. Hepatology. 2010;52(5):1600–10.
Roznovsky L, Harrison TJ, Fang ZL, Ling R, Lochman I, Orsagova I, et al. Unusual hepatitis B surface antigen variation in a child immunised against hepatitis B. J Med Virol. 2000;61(1):11–4.
Liu Y, Ye S, Xiao X, Zhou T, Yang S, Wang G, et al. Association of diabetes mellitus with hepatitis B and hepatitis C virus infection: evidence from an epidemiological study. Infect Drug Resist. 2019;12:2875–83.
World Health Organization. Diabetes, WHO fact sheet. 2021. https://www.who.int/news-room/fact-sheets/detail/diabetes.
Farshadpour F, Taherkhani R, Ravanbod MR, Eghbali SS. Prevalence and genotype distribution of hepatitis C virus infection among patients with type 2 diabetes mellitus. Med Princ Pract. 2018;27(4):308–16.
Hong YS, Chang Y, Ryu S, Cainzos-Achirica M, Kwon MJ, Zhang Y, et al. Hepatitis B and C virus infection and diabetes mellitus: a cohort study. Sci Rep. 2017;7(1):4606.
Cai C, Zeng J, Wu H, Shi R, Wei M, Gao Y, et al. Association between hepatitis B virus infection and diabetes mellitus: a meta-analysis. Exp Ther Med. 2015;10(2):693–8.
Lei S, Chen S, Zhao X, Zhang Y, Cheng K, Zhang X, et al. Hepatitis B virus infection and diabetes mellitus: the Kailuan prospective cohort study in China. Hepatol Int. 2020;14(5):743–53.
Zhang X, Zhu X, Ji Y, Li H, Hou F, Xiao C, et al. Increased risk of hepatitis B virus infection amongst individuals with diabetes mellitus. Biosci Rep. 2019;39(3):1–7.
Mirzaei M, Rahmaninan M, Mirzaei M, Nadjarzadeh A, Dehghani Tafti AA. Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes in Central Iran: results from Yazd health study. BMC Public Health. 2020;20(1):166.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Med. 1998;15(7):539–53.
Sitnik R, Pinho JR, Bertolini DA, Bernardini AP, Da Silva LC, Carrilho FJ. Hepatitis B virus genotypes and precore and core mutants in Brazilian patients. J Clin Microbiol. 2004;42(6):2455–60.
Chen J, Yin J, Tan X, Zhang H, Zhang H, Chen B, et al. Improved multiplex-PCR to identify hepatitis B virus genotypes A–F and subgenotypes B1, B2, C1 and C2. J Clin Virol. 2007;38(3):238–43.
Taherkhani R, Farshadpour F. Prevalence, genotype distribution and mutations of hepatitis B virus and the associated risk factors among pregnant women residing in the northern shores of Persian Gulf, Iran. PLoS ONE. 2022;17(3): e0265063.
Italiano CM, Speers DJ, Chidlow GR, Dowse GK, Robertson AG, Flexman JP. Hepatitis B outbreak following a mass-casualty incident, Australia. J Infect Dis. 2011;204(3):400–7.
Boot HJ, Cremer J, Koedijk FD, van Ballegooijen WM, Op de Coul EL. Improved tracing of hepatitis B virus transmission chains by phylogenetic analysis based on C region sequences. J Med Virol. 2008;80(2):233–41.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4.
Taherkhani R, Farshadpour F, Makvandi M, Hamidifard M, Esmailizadeh M, Ahmadi B, et al. Determination of cytomegalovirus prevalence and glycoprotein B genotypes among ulcerative colitis patients in Ahvaz, Iran. Jundishapur J Microbiol. 2015;8(2): e17458.
Farshadpour F, Taherkhani R, Tajbakhsh S, Gholizadeh Tangestani M, Hajiani G, Sharifi N, et al. Prevalence and trends of transfusion-transmissible viral infections among blood donors in south of Iran: an eleven-year retrospective study. PLoS ONE. 2016;11(6): e0157615.
Mohammadi Z, Keshtkar A, Eghtesad S, Jeddian A, Pourfatholah AA, Maghsudlu M, et al. Epidemiological profile of hepatitis B virus infection in Iran in the past 25 years; a systematic review and meta-analysis of general population studies. Middle East J Dig Dis. 2016;8(1):5–18.
Tan Y, Wei S, Zhang W, Yang J, Yang J, Yan L. Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: a meta-analysis and systematic review. Cancer Manag Res. 2019;11:705–13.
Fu SC, Huang YW, Wang TC, Hu JT, Chen DS, Yang SS. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with new onset diabetes: a nationwide cohort study. Aliment Pharmacol Ther. 2015;41(11):1200–9.
Spradling P, Simons B, Narayanan M, Xing J, Homan C, Bulkow L, et al. Incidence of diabetes mellitus in a population-based cohort of persons with chronic hepatitis B virus infection. J Viral Hepat. 2013;20(7):510–3.
Soverini V, Persico M, Bugianesi E, Forlani G, Salamone F, Masarone M, et al. HBV and HCV infection in type 2 diabetes mellitus: a survey in three diabetes units in different Italian areas. Acta Diabetol. 2011;48(4):337–43.
Merza MA. Seroprevalence and risk factors of hepatitis B and C viruses among diabetes mellitus patients in Duhok province, Iraqi Kurdistan. J Fam Med Prim Care. 2020;9(2):642–6.
Mekonnen D, Gebre-Selassie S, Fantaw S, Hunegnaw A, Mihret A. Prevalence of hepatitis B virus in patients with diabetes mellitus: a comparative cross sectional study at Woldiya General Hospital, Ethiopia. Pan Afr Med J. 2014;17(1):40.
Lu J, Hou X, Tu H, Tang Z, Xiang Y, Bao Y, et al. Chronic hepatitis B virus infection status is more prevalent in patients with type 2 diabetes. J Diabetes Investig. 2017;8(4):619–25.
Chen HF, Li CY, Chen P, See TT, Lee HY. Seroprevalence of hepatitis B and C in type 2 diabetic patients. J Chin Med Assoc. 2006;69(4):146–52.
Schillie S, Xing J, Murphy T, Hu D. Prevalence of hepatitis B virus infection among persons with diagnosed diabetes mellitus in the United States, 1999–2010. J Viral Hepat. 2012;19(9):674–6.
Arrelias CCA, Rodrigues FB, Torquato MTdCG, Teixeira CRdS, Rodrigues FFL, Zanetti ML. Prevalence of serological markers for hepatitis and potential associated factors in patients with diabetes mellitus. Rev Lat Am Enfermagem. 2018;26:e3085.
Chachá SGF, Gomes-Gouvêa MS, Malta FdM, Ferreira SdC, Villanova MG, Souza FF, et al. Basal core promoter and precore mutations among hepatitis B virus circulating in Brazil and its association with severe forms of hepatic diseases. Mem Inst Oswaldo Cruz. 2017;112(9):626–31.
Dooghaie Moghadam A, Eslami P, Dowlati Beirami A, Iravani S, Farokhi E, Mansour-Ghanaei A, et al. An overview of the current hepatitis B treatment strategies after liver transplantation. Middle East J Dig Dis. 2021;13(1):5–14.
Khodadad N, Seyedian SS, Haghighi SB, Makvandi M. Molecular characterization and phylogenetic analyses of full-length viral genomes from Iranian patients with chronic hepatitis B virus. Future Virol. 2021;16(10):667–75.
Sunbul M. Hepatitis B virus genotypes: global distribution and clinical importance. World J Gastroenterol. 2014;20(18):5427–34.
Lin CL, Kao JH. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med. 2015;5(5):a021436.
Yano Y, Azuma T, Hayashi Y. Variations and mutations in the hepatitis B virus genome and their associations with clinical characteristics. World J Hepatol. 2015;7(3):583–92.
Acknowledgements
The authors would like to acknowledge grant number 3254 supported by the Deputy Research and Affairs of the Bushehr University of Medical Sciences, Bushehr, Iran.
Funding
This study was funded by the Bushehr University of Medical Sciences with Grant Number 3254. The institutional grant only includes the financial assistance to meet all expenses, materials used, and essential equipment for conducting the study. The funder had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
FF and RT designed and performed the study. FS performed the study. RT and FF drafted and edited the manuscript. All authors approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the Bushehr University of Medical Sciences with reference number IR.BPUMS.REC.1395.54. All of the participants have signed informed consent forms to use their serum samples for HBV detection and analysis. All methods were performed under the relevant guidelines and regulations.
Consent for publication
All of the participants gave written informed consent to publish their data in a journal article.
Competing interests
The authors declared that they do not have anything to disclose regarding conflict of interest concerning this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Prevalence of HbsAg, HBcAb and HBV viremia in diabetics patients and non-diabetic controls. Table S2. Prevalence of HBsAg according to demographic and biochemical variables among diabetic patients. Table S3. Prevalence of HBcAb according to demographic characteristics among diabetic patients. Table S4. Prevalence of HBsAg according to demographic characteristics among non-diabetic controls. Table S5. Prevalence of HBcAb according to demographic characteristics among non-diabetic controls. Table S6. Mutations in HBV genome. Figure S1. The PCR amplification of the S region of HBV genome extracted from the serum samples of diabetic patients. L, 100-bp DNA ladder; N, negative control; P, positive control; 2-7, 9-11, 14 and 19, amplified product (≈417 bp) on 2% agarose gel electrophoresis. Figure S2. The PCR amplification of the X and pre-core regions of HBV genome extracted from the serum samples of diabetic patients. L, 100-bp DNA ladder; N, negative control; P, positive control; 2–7, amplified product (≈735 bp) on 2% agarose gel electrophoresis. Figure S3. Alignment of the amino acid sequences of HBsAg (64 aa to 173 aa) of strains isolated from the diabetic patients (GenBank accession Nos. MF419214–MF419229) and the reference sequences available at the nucleotide database of the NCBI. Figure S4. Alignment of amino acid sequences of the pre-core region isolated from the diabetic patients (GenBank accession Nos. OK382075-OK382085) and the reference sequences available at the nucleotide database of the NCBI. Figure S5. Alignment of 109 to 154 amino acid sequences of the X protein (1698 to 1838 nucleotide sequence) of strains isolated from the diabetic patients (GenBank accession Nos. OK382075-OK382085) and the reference sequences available at the nucleotide database of the NCBI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Farshadpour, F., Taherkhani, R. & Saberi, F. Molecular evaluation of hepatitis B virus infection and predominant mutations of pre-core, basal core promoter and S regions in an Iranian population with type 2 diabetes mellitus: a case–control study. BMC Infect Dis 22, 553 (2022). https://doi.org/10.1186/s12879-022-07528-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07528-7