Abstract
Background
The empirical prescription of antibiotics to inpatients with Coronavirus Disease 2019 (COVID-19) is frequent despite uncommon bacterial coinfections. Current knowledge of the effect of antibiotics on the survival of hospitalized children with COVID-19 is limited.
Objective
To characterize the survival experience of children with laboratory-positive COVID-19 in whom antibiotics were prescribed at hospital admission.
Methods
A retrospective cohort study was conducted in Mexico, with children hospitalized due to COVID-19 from March 2020 to December 2021. Data from 1601 patients were analyzed using the Kaplan–Meier method and the log-rank test. We computed hazard ratios (HR) and 95% confidence intervals (CI) to evaluate the effect of the analyzed exposures on disease outcomes.
Results
Antibiotics were prescribed to 13.2% (\(n\) = 211) of enrolled children and a higher mortality rate [14.9 (95% CI 10.1–19.8) vs. 8.3 (95% CI 6.8–9.8)] per 1000 person-days, \(p\) < 0.001) was found among them. At any given cut-off, survival functions were lower in antibiotic-positive inpatients (\(p\) < 0.001). In the multiple model, antibiotic prescription was associated with a 50% increase in the risk of fatal outcome (HR = 1.50, 95% CI 1.01–2.22). A longer interval between illness onset and healthcare-seeking and pneumonia at hospital admission was associated with a poorer prognosis.
Conclusions
Our results suggest that antibiotic prescription in children hospitalized due to COVID-19 is associated with decreased survival. If later replicated, these findings highlight the need for rational antibiotics in these patients.
Similar content being viewed by others
Background
The Coronavirus Disease 2019 (COVID-19) burden among children in Mexico has been high [1]. By mid-January 2022, and among children aged 9 years or younger, more than 85 thousand confirmed cases of COVID-19 had been registered, together with 7.3 thousand hospital admissions [2].
Latin-American countries had been hard-hit by the COVID-19 pandemic. In the region, the cumulative mortality rate (per 100 thousand people) observed in Mexico by the start of April 2022 (254) is only overcome by Peru (654), Brazil (314), Chile (302), Argentina (286), Colombia (278) and Paraguay (267) [3].
Current knowledge on managing adult or children patients with COVID-19 is insufficient [4]. Despite bacterial coinfections being infrequent and presented only in around 8% of patients [5], the empirical use of antibiotics in patients with COVID-19 has been widely documented since the start of the epidemic in the city of Wuhan, China [6].
The proportion of COVID-19 Chinese patients receiving antibiotics during hospital stay was around 50% [7, 8]. In general, higher rates have been registered in the United States and European countries, above 70% [9, 10].
Antibiotic prescription rates as high as 86% have been documented in hospitalized children [11]. This is particularly concerning since a poorer survival has been reported among COVID-19 adult inpatients receiving antibiotics [10]; moreover, to the best of our knowledge, there are not hitherto published studies evaluating in-hospital outcomes in children patients receiving antibiotics. This study aimed to characterize the survival experience of children hospitalized due to a laboratory-positive COVID-19 result in whom antibiotics were prescribed.
Methods
Study design and setting
We conducted a nationwide retrospective cohort study in Mexico from November 2021 to January 2022. Children that were hospitalized due to laboratory-confirmed (reverse-transcription polymerase chain reaction, RT-PCR) COVID-19 were potentially eligible. They were identified from the nominal records found in a national and normative system for epidemiological surveillance of respiratory viruses, which belongs to the Mexican Institute of Social Security (IMSS, the Spanish acronym). IMSS is part of the public healthcare system. It provides medical assistance, social protection, and integral services to its users through more than 6.5 thousand medical units (350 and 36 of them being secondary and tertiary care hospitals, respectively) located across Mexico.
Study population
According to normative standards, RT-PCR testing is performed in all suspected cases of COVID-19 requiring hospital admission [12]. Hospitalized children aged 9 years or younger, with the onset of symptoms of COVID-19 from March 2020 to December 2021 and with conclusive test results, were eligible. Individuals with missing clinical or epidemiologic data of interest were excluded.
Data collection
Data of interest were retrieved from the audited surveillance system, which primary data sources were the medical records of enrolled patients and death certificates, if applicable. Analyzed information included demographic characteristics (sex, age), personal history of noncommunicable diseases (no/yes: obesity, type 1 diabetes mellitus, asthma, chronic kidney disease, immunosuppression or cardiovascular disease), administration of antibiotics (any; no/yes) at hospital admission, clinical manifestations (no/yes: fever or chills, cough, shortness of breath, and tachypnea) and pneumonia-related radiographic findings (no/yes: ground glass patterns in X-ray or computed tomography scanning). Hospitalized children with pneumonia at admission were those with both clinical manifestations and radiographic findings of this abnormality [13].
Date of healthcare-seeking and dates of hospital admission and discharge (and the causes of hospital discharge [recovery/death], were also extracted from the audited database. The interval (days) elapsed between the symptoms onset and the date of healthcare-seeking were computed.
We used the date of symptom onset as an approximation for the SARS-COV-2 variant causing the infection and were categorized as March 2020–April 2021 or May 2021–December 2021, when the dominant variants were the ancestral and Delta (B.1.617.2), respectively [14].
Outcome
The primary outcome was the cause [recovery or death (due to any immediate cause)] of hospital discharge of children hospitalized due to COVID-19.
Laboratory methods
Clinical specimens (deep nasal swabs) were analyzed (SuperScript™ III Platinum™ One-Step qRT-PCR Kits) at four specialized regional laboratories integrated into the IMSS network for epidemiologic surveillance. A broader description of the laboratory methods has already been published elsewhere [15].
Statistical analysis
We computed summary statistics and the significance level (α) at 5%. The Kaplan–Meier method calculated survival functions and 95% confidence intervals (CI). The log-rank test was used to compare the survival distributions of the study groups. The effect of the prescription of antibiotics (any) was evaluated through hazard ratios (HR), and 95% CI was computed using a multivariate Cox proportional hazard regression model. All analyses were conducted using Stata version 16.0 (StataCorp; College Station, TX, USA).
Results
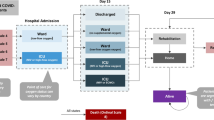
Data from 1601 patients were analyzed for a total follow-up of 16,238 person-days. Figure 1 shows the study profile. Antibiotic prescription was identified in 13.2% (\(n\) = 211/1601; 95% CI 11.6–14.9%) of analyzed inpatients and the overall frequency, according to disease outcome, was 12.1% (95% CI 10.5–13.9%) vs. 23.8% (95% CI 17.0–30.6%) in non-fatal and fatal cases respectively (\(p\) < 0.001). Cephalosporins were the most commonly prescribed antibiotics.
The overall rate of a fatal in-hospital outcome was 9.3 (\(n\) = 151/16,238; 95% 7.8–10.8) per 1000 person-days. The mortality rate was higher among antibiotic-positive inpatients [14.9 (95% CI 10.1–19.8) vs. 8.3 (95% CI 6.8–9.8) per 1000 person-days, \(p\) < 0.001]. Table 1 summarizes the characteristics of participants for selected variables.
As presented in Fig. 2, survival rates were lower among patients prescribed antibiotics, specifically on day 15 of hospital admission and later (log-rank test, p = 0.001). The specifics survival rates (antibiotics were prescribed, yes vs. no), according to the number of days elapsed since hospital entry, were as follows: 1 day, 98.0% (95% CI 94.8–99.3%) vs. 98.8% (95% CI 98.0–99.3%); 3 days, 96.9% (95% CI 93.2–98.6%) vs. 97.7% (95% CI 96.7–98.4%); 7 days, 91.0% (95% CI 85.6–94.5%) vs. 95.2% (95% CI 93.8–96.4%); 15 days, 82.3% (95% CI 74.9–87.7%) vs. 90.1% (95% CI 87.9–91–9%); 21 days, 77.6% (95% CI 69.6–83.8%) vs. 88.8% (95% CI 86.4–90.6%); and 30 days, 73.4% (95% CI 64.9–80.2%) vs. 86.5% (95% CI 83.8–88.7%).
In the multiple regression model (Table 2), and compared with children who did not receive antibiotics, hospitalized children receiving any of these drugs had a 50% increased risk of fatal outcome (HR = 1.50, 95% CI 1.01–2.22). We also documented a poorer in-hospital outcome in patients with more days elapsed between illness onset and healthcare-seeking (per each additional day: HR = 1.05, 95% CI 1.01–1.10), as well as a nearly twofold increase in the risk of death among children with pneumonia at hospital entry (HR = 1.94, IC 95% 1.37–2.77).
Discussion
We characterized the survival experience of a large subset of children inpatients with laboratory-confirmed COVID-19. Our findings suggest that prescribing antibiotics to pediatric COVID-19 patients is associated with a poorer in-hospital prognosis. However, given the limitations of an observational study, the results must be carefully considered.
Published data regarding the antibiotic prescription rates in hospitalizes are scarce, and the estimates heterogeneous. In our study, these drugs were prescribed to around 13% of enrolled inpatients. This frequency is almost a half (24.5%, p < 0.001) of the rate documented by a previously published Latin-American multicenter study where most of the analyzed patients were from Peru and Costa Rica [16]. Our rate is also lower than the computed by a metanalysis where 154 studies were analyzed (38.5%, 95% 26.3–52.3%; p < 0.001) [9].
We hypothesize that two factors may be determined, at least partially, by the low rates of antibiotic prescription documented in our study. The first is that all hospitals from where patients were recruited were public settings belonging to IMSS. For-profit hospitals have been associated with a higher risk of receiving these drugs [17]. The second factor is that IMSS developed early (April 2020) protocols for the attention of COVID-19 patients [18].
The main factors determining the start of antibiotics seem to be increased inflammatory markers, and any infiltrate on an x-ray image [19]. In our study sample, children inpatients with pneumonia were more likely to receive antibacterial drugs at admission (31.1% vs. 9.1%, p < 0.001).
The empirical prescription of antibiotics in COVID-19 patients does not reduce the risk for severe symptoms or death [20]. The clinical usefulness of macrolides, for their anti-inflammatory properties, is also questionable [21, 22].
The overuse of antibiotics in patients with COVID-19, especially combinations of broad-spectrum antibiotics, has become a significant concern [23]. Fighting the threat of antibiotic resistance is a public health priority as crucial to limiting the spread of SARS-COV-2, especially in children with a high respiratory infection rate [24].
The potential limitations of our study must be cited. First, data regarding the prescription of antibiotics were collected as a dichotomous variable, and other clinical and epidemiological relevant information (such as length of administration) was omitted. We also documented that in around 40% of inpatients, the precisely prescribed antibiotic was not registered. Second, we did not evaluate intermediate outcomes such as the multisystem inflammatory syndrome temporally related to COVID-19 [25], which might impact antibiotic prescription rates. Third, the initial symptoms of COVID-19 may be unspecific [26]. Therefore, we recommend carefully considering our slight but significant increased risk of death among children with more days from symptom onset to healthcare seeking. Finally, we used anonymized data, and we were unable to identify what patients received medical care in secondary and tertiary care hospitals. The latter may have had any effect on the observed estimates. However, above 90% of units from which patients were recruited were secondary care hospitals.
Conclusions
Our findings suggest that antibiotic prescription in children inpatients with COVID-19 is associated with an increased risk for fatal outcome. If later replicated in other populations, our results highlight the major relevance of limiting the empirical administration of anti-bacterial drugs in these patients.
Availability of data and materials
All data generated or analyzed during this study are included within this published article.
References
Rivas-Ruiz R, et al. Factors associated with death in children with COVID-19 in Mexico. Gac Med Mex. 2020;156(6):516–22.
Government of Mexico. COVID-19 in Mexico. General data [updated 2022 Jan 20]. Accessed 21 Jan 2022. [Webpage in Spanish].
Johns Hopkins, University of Medicine. Coronavirus resource center: mortality analyses [Updated 2022 Apr 21]. Available at: https://coronavirus.jhu.edu/data/mortality. Accessed 21 Apr 2022.
Mega C, et al. Protective ventilation in patients with acute respiratory distress syndrome related to COVID-19: always, sometimes or never? Curr Opin Crit Care. 2022;28(1):51–6.
Rawson TM, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–68.
Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Zhang J, et al. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8(3):e11–2.
Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Langford BJ, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–9.
Beovic B, et al. Antibiotic use in patients with COVID-19: a ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J Antimicrob Chemother. 2020;75(11):3386–90.
Chowdhury K, et al. Management of children admitted to hospitals across Bangladesh with suspected or confirmed COVID-19 and the implications for the future: a nationwide cross-sectional study. Antibiotics (Basel). 2022;11(1):105.
Health Ministry and General Directorate of Epidemiology of Mexico. Standardized guidelines for the epidemiological and laboratory surveillance of viral respiratory diseases [Updated 2022 Jan 19]. Available at: https://coronavirus.gob.mx/wp-content/uploads/2022/01/2022.01.12-Lineamiento_VE_ERV_DGE.pdf. Accessed 27 Jan 2022.
Government of Mexico. Clinical guidelines for the treatment of COVID-19 in Mexico [Updated 2021 Aug 2]. Available at: https://coronavirus.gob.mx/wp-content/uploads/2021/08/GuiaTx_COVID19_ConsensoInterinstitucional_2021.08.03.pdf. Accessed 03 Feb 2022. [Webpage in Spanish].
General Directorate of Epidemiology of Mexico. Genomic surveillance report of the SARS-CoV-2 virus in Mexico. National and state distribution of variants as of April 04, 2022. Available at: https://coronavirus.gob.mx/wp-content/uploads/2022/04/2022.04.04-Varientes-COVID-MX.pdf. Accessed 22 Apr 2022. [Online document in Spanish].
Murillo-Zamora E, et al. Male gender and kidney illness are associated with an increased risk of severe laboratory-confirmed coronavirus disease. BMC Infect Dis. 2020;20(1):674.
Yock-Corrales A, et al. High rates of antibiotic prescriptions in children with COVID-19 or multisystem inflammatory syndrome: a multinational experience in 990 cases from Latin America. Acta Paediatr. 2021;110(6):1902–10.
Vaughn VM, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72(10):e533–41.
Mexican Institute of Social Security. Guidelines for the attention of SARS-CoV-2 (COVID-19) patients (April 2020). Available at: http://www.imss.gob.mx/sites/all/statics/imssBienestar/marcoJuridico/Guia-Pacientes-COVID19.pdf. Accessed 22 Apr 2022. [Online document in Spanish].
Bendala Estrada AD, et al. Inadequate use of antibiotics in the covid-19 era: effectiveness of antibiotic therapy. BMC Infect Dis. 2021;21(1):1144.
Popp M, et al. Antibiotics for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;10:CD015025.
Naja M, Wedderburn L, Ciurtin C. COVID-19 infection in children and adolescents. Br J Hosp Med (Lond). 2020;81(8):1–10.
Poddighe D, Aljofan M. Clinical evidences on the antiviral properties of macrolide antibiotics in the COVID-19 era and beyond. Antivir Chem Chemother. 2020;28:2040206620961712.
Wang J, et al. Efficacy and safety of antibiotic agents in children with COVID-19: a rapid review. Ann Transl Med. 2020;8(10):619.
Borrelli M, et al. Coronavirus disease 2019 in children. Front Pediatr. 2021;9: 668484.
Feldstein LR, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–46.
Baj J, et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9(6):1753.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
EMZ conceived and designed the experiments and also wrote the first draft of the manuscript; XT performed the data analysis and collection, MH contributed with the Methodology and Writing—review and editing; MRS and ALR contributed with revisions and data analysis and OMC performed the experiments, analyzed the data, and is responsible for the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Local Research Ethics Committee (601) of the IMSS approved this study (Approval ID: R-2020-601-015). All the legal guardians provided the informed written consent for the use of anonymized data from their children for the purposes of this research. We confirm that all experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Murillo-Zamora, E., Trujillo, X., Huerta, M. et al. Decreased survival in children inpatients with COVID-19 and antibiotic prescription. BMC Infect Dis 22, 532 (2022). https://doi.org/10.1186/s12879-022-07516-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07516-x