Abstract
Background
Legionella spp. is recognized as a common cause of community acquired pneumonia, with Legionella pneumophila serogroup 1 being the most prevalent. At least 70 species are described so far but few are identified in pathogenic conditions. Data on extrapulmonary infections are scarce.
Case presentation
A 73-yar-old male with chronic lymphoid leukemia was hospitalized for an insidious wrist arthritis. Ultrasound of the wrist showed a carpal and radiocarpal fluid effusion with positive Doppler signal. While routine bacterial cultures remained sterile, 16S rRNA PCR identified Legionella anisa. Ciprofloxacin 500 mg twice a day for a period of six weeks improved arthritis with full recovery at the end of the treatment.
Conclusion
Legionella non pneumophila are a rare cause of septic arthritis especially found in immunosuppressed patients and identification of species could help clinician to adapt antibiotherapy.
Similar content being viewed by others
Background
Legionella spp. is recognized as a common cause of community acquired pneumonia, with Legionella pneumophila serogroup 1 being the most prevalent. 70 species are described so far but few are identified in pathogenic conditions [1]. Data on extrapulmonary infections are scarce. Herein, we report a case of Legionella anisa monoarthritis.
Case presentation
A 73-year-old male was hospitalized in the rheumatology department for an insidious inflammatory swelling of the right wrist. Symptoms began six weeks before with a localized swelling of the right index finger. He received NSAID followed by a week of pristinamycin without improvement. He reported no local trauma, respiratory symptoms or fever but occasional mild night sweats.
He had a medical history of chronic lymphoid leukemia (CLL), treated by chemotherapy five years before (bendamustine in association with rituximab). He was a former postman and had gardening and woodworking as hobbies.
On admission, the patient showed right wrist synovitis since two months without extra rheumatologic complaints. Blood tests showed leukocytosis (40.8 G/L) with lymphocyte predominance (32 G/L). Neutrophil count was also increased (7.6 G/L), as well as C-reactive protein (44 mg/L). Liver enzymes were within ranges. Immunological assays were negative, including rheumatoid factor and anti-CCP antibodies (except anti-nuclear antibodies at 1/160, without specificity). There was no hypogammaglobulinemia.
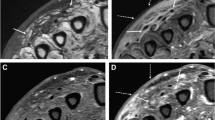
Ultrasound of the wrist showed a carpal and radiocarpal fluid effusion with positive Doppler signal (Fig. 1). There was a palmar and dorsal subcutaneous infiltration as well, without collection. Fluid aspiration was hemorrhagic, with a white blood count of 36900/mm3. Neutrophil count was 43% and mono-histiocytes count was 49%. Routine bacterial cultures remained sterile but 16S ribosomal RNA (rRNA) polymerase chain reaction (PCR) identified Legionella anisa at day 4. The manufacturer of the equipment was Diagenode. Blood cultures were sterile.
Ciprofloxacin 500 mg twice a day for a period of six weeks improved arthritis with full recovery at the end of the treatment. Interestingly, C-reactive protein showed spontaneous normalization before any treatment.
The source of infection was presumably gardening. The patient had a well in his garden. Chest X-ray was normal. No environmental exploration was performed according to the national reference center guidelines.
Discussion and conclusion
Legionella spp. are ubiquitous, aerobic, gram-negative rods naturally found in freshwater environments and are usually transmitted to humans in aerosols. They are regarded as fastidious bacteria as they do not grow on routine bacteriologic media. The clinical manifestations of Legionella infections are primarily respiratory (Legionnaires’ disease), but several extrapulmonary infections has been described. Legionnella spp has been implicated in arthritis, meningitis, sinusitis, endocarditis, pericarditis, myocarditis, pancreatitis, peritonitis and soft tissue infections [2].
While L. pneumophila is responsible to the vast majority of human infections, data on L. anisa pathogenicity are scarce. Despite being one of the most frequent species of Legionella in the environment, only eight articles reported infections secondary to L. anisa [3,4,5,6,7,8,9,10] (Table 1). In a French study, this strain was the most frequent non-pneumophila species in the environment (13.8%), but only accounted for 0.8% of the clinical isolates [11]. It has been responsible of hospital water system contamination, as well as nosocomial infections. Besides, there are concerns that L. anisa could mask L. pneumophila water contamination [12]. Clinical manifestations described are mainly respiratory with eight reported pneumonia (seven immunocompromised (IC) patients) [8, 9] and 34 Pontiac fever during an outbreak in California [10]. Other manifestations included one pleural infection with probable pneumonia (IC) [5], one osteomyelitis secondary to pneumonia (IC) [4], one chronic endocarditis [6] and one mycotic aortic aneurysm [3] in both immunocompetent patients.
Immunologic response to Legionella infection is complex. L. pneumophila activates an important inflammatory response in hosts, with innate and adaptive responses. IFN-γ and TNFα are primarily responsible for immune clearance while CD4 + and CD8 + T cells additionally contribute to host defense [13]. Humoral response is considered feeble and does not provide prolonged immunity against the pathogen.
Arthritis caused by Legionella spp are rare, with only twelve cases previously described (Table 2). Seven were immunocompromised and two had kidney insufficiency (one moderate and one presumably non-severe given the arthritis antibiotic management). Median age at diagnosis was 71, range (51–90). Inoculation occurred most frequently through skin wound which are nonetheless rarely found at diagnosis. Some reports mentioned potential inoculation through corticosteroid injections [14,15,16]. However, acute arthritis following such injection could be unrecognized legionella infection potentiated by the induced local immunosuppression. Final, reactive arthritis has been a concern in one article and present with positive 16S RNA PCR with inflammatory fluid [17].
The patients often presented few symptoms amid localized pain. Fever is rarely described (two cases with polyarthritis) [18, 19]. Delayed diagnosis is frequent with a median of 21 days, range (2–90). Polyarthritis seems to be a concern of L. pneumophila serogroup 1 (Lp1). Non-pneumophila strains are more frequently isolated in monoarthritis which is consistent with the direct mode of transmission [20]. Blood samples usually showed increase C-reactive protein, median 147 mg/L, range (< 5–254 mg/L). Fluid aspirate was hemorrhagic in two cases [20, 21], as our patient, with median neutrophil count of 80%, range (23–90).
Patients with significant immunosuppression (no isolated humoral deficiency as discussed previously) were older (median 80 vs 56 years) and had longer delayed diagnosis (median 32 vs 16 days).
Diagnosis was performed by 16S RNA PCR in each case except three. The other means of diagnosis were urinary antigen test for Lp1, serology, NGS and cultures. Legionella spp. require non-routine culture media for growth, especially BCYE. Successful cultures with chocolate agar and mycobacteria specific medium have been reported [22, 23]. Microbiologist must be aware of Legionella suspicion to perform such culture, which may lead to under-recognize diagnosis. Wide spreading of PCR might fill this gap. MALDI-TOF can be helpful for species identification [24].
There is no standard for antimicrobial therapy. Treatment consisted of fluoroquinolones in the majority of cases (9/11). Five patients had combination therapy (four rifampicin, one azithromycin). Data was missing in one patient. Median duration of antibiotic therapy for native septic arthritis was 42 days, range (21–90). One patient with knee prosthesis infection and was successfully treated with levofloxacin and rifampicin for five months. All strategies were effective.
We present the first case of septic arthritis caused by L. anisa. Legionella spp. should be suspected in arthritis, especially involving extremities and knee, with sterile standard culture, insidious evolution and compatible exposition. Concomitant pneumonia is uncommon but immunosuppression is not. Older age is probably a risk factor for Legionella arthritis.
Availability of data and materials
Not applicable.
Abbreviations
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- RNA:
-
Ribonucleic acid
- PCR:
-
Polymerase chain reaction
- NGS:
-
Next generation sequencing
- BCYE:
-
Buffered charcoal yeast extract
- MALDI–TOF:
-
Matrix assisted laser desorption ionisation/time of flight
- F:
-
Female
- M:
-
Male
- LNZ:
-
Linezolid
- PFX:
-
Pazufloxacin
- LFX:
-
Lefofloxacin
- CTM:
-
Clarythromycin
- MFX:
-
Moxifloxacin
- ERM:
-
Erythromycin
- CFX:
-
Cefixime
- CPX:
-
Ciprofloxacin
- NA:
-
Not available
- L:
-
Left
- R:
-
Right
- CS:
-
Corticosteroids
- MTX:
-
Methotrexate
- TCZ:
-
Tocilizumab
- UAT:
-
Urinary antigen test
- AZM:
-
Azithromycin
- RFP:
-
Rifampicin
References
Cunha BA, Burillo A, Bouza E. Legionnaires’ disease. Lancet. 2016;387(10016):376–85.
Lowry PW, Tompkins LS. Nosocomial legionellosis: a review of pulmonary and extrapulmonary syndromes. Am J Infect Control. 1993;21(1):21–7.
Tanabe M, Nakajima H, Nakamura A, Ito T, Nakamura M, Shimono T, et al. Mycotic aortic aneurysm associated with Legionella anisa. J Clin Microbiol. 2009;47(7):2340–3.
Sanchez MC, Sebti R, Hassoun P, Mannion C, Goy AH, Feldman T, et al. Osteomyelitis of the patella caused by Legionella anisa. J Clin Microbiol. 2013;51(8):2791–3.
Bornstein N, Mercatello A, Marmet D, Surgot M, Deveaux Y, Fleurette J. Pleural infection caused by Legionella anisa. J Clin Microbiol. 1989;27(9):2100–1.
Compain F, Bruneval P, Jarraud S, Perrot S, Aubert S, Napoly V, et al. Chronic endocarditis due to Legionella anisa: a first case difficult to diagnose. N Microb N Infect. 2015;8:113–5.
Thacker WL, Benson RF, Hawes L, Mayberry WR, Brenner DJ. Characterization of a Legionella anisa strain isolated from a patient with pneumonia. J Clin Microbiol. 1990;28(1):122–3.
Vaccaro L, Izquierdo F, Magnet A, Hurtado C, Salinas MA, Gomes TS, et al. First case of Legionnaire’s disease caused by Legionella anisa in Spain and the limitations on the diagnosis of Legionella non-pneumophila infections. PLoS ONE. 2016;11(7):e0159726.
Head BM, Trajtman A, Bernard K, Burdz T, Vélez L, Herrera M, et al. Legionella co-infection in HIV-associated pneumonia. Diagn Microbiol Infect Dis. 2019;95(1):71–6.
Fenstersheib MD, Miller M, Diggins C, Liska S, Detwiler L, Werner SB, et al. Outbreak of pontiac fever due to Legionella anisa. Lancet. 1990;336(8706):35–7.
Doleans A, Aurell H, Reyrolle M, Lina G, Freney J, Vandenesch F, et al. Clinical and environmental distributions of Legionella strains in France are different. J Clin Microbiol janv. 2004;42(1):458–60.
van der Mee-Marquet N, Domelier AS, Arnault L, Bloc D, Laudat P, Hartemann P, et al. Legionella anisa, a possible indicator of water contamination by Legionella pneumophila. J Clin Microbiol. 2006;44(1):56–9.
Chauhan D, Shames SR. Pathogenicity and virulence of legionella: intracellular replication and host response. Virulence. 2021;12(1):1122–44.
Banderet F, Blaich A, Soleman E, Gaia V, Osthoff M. Septic arthritis due to Legionella cincinnatiensis: case report and review of the literature. Infection. 2017;45(4):551–5.
Flendrie M, Jeurissen M, Franssen M, Kwa D, Klaassen C, Vos F. Septic arthritis caused by Legionella dumoffii in a patient with systemic lupus erythematosus-like disease. J Clin Microbiol févr. 2011;49(2):746–9.
Fernández-Cruz A, Marín M, Castelo L, Usubillaga R, Martín-Rabadán P, Bouza E, et al. Legionella micdadei, a new cause of prosthetic joint infection. J Clin Microbiol. 2011;49(9):3409–10.
Naito T, Suda T, Saga K, Horii T, Chida K. Reactive Legionella pneumophila arthritis diagnosed by polymerase chain reaction. Rheumatol Int. 2007;27(4):415–6.
Naito T, Suda T, Saga K, Horii T, Chida K. Reactive Legionella pneumophila arthritis diagnosed by polymerase chain reaction. Rheumatol Int févr. 2007;27(4):415–6.
Dugar M, Rankin WA, Rowe E, Smith MD. «My foot hurts»: a flare of rheumatoid arthritis? Med J Aust. 2009;190(7):392–3.
Ibranosyan M, Beraud L, Lemaire H, Ranc AG, Ginevra C, Jarraud S, et al. The clinical presentation of Legionella arthritis reveals the mode of infection and the bacterial species: case report and literature review. BMC Infect Dis. 2019;19(1):864.
Huang Y, Ma Y, Miao Q, Pan J, Hu B, Gong Y, et al. Arthritis caused by Legionella micdadei and Staphylococcus aureus: metagenomic next-generation sequencing provides a rapid and accurate access to diagnosis and surveillance. Ann Transl Med. 2019;7(20):589.
Bemer P, Leautez S, Ninin E, Jarraud S, Raffi F, Drugeon H. Legionella pneumophila arthritis: use of medium specific for mycobacteria for isolation of L. pneumophila in culture of articular fluid specimens. Clin Infect Dis. 2002;35(1):6–7.
Linscott AJ, Poulter MD, Ward K, Bruckner DA. Legionella pneumophila serogroup 4 isolated from joint tissue. J Clin Microbiol mars. 2004;42(3):1365–6.
Just SA, Knudsen JB, Uldum SA, Holt HM. Detection of Legionella bozemanae, a new cause of septic arthritis, by PCR followed by specific culture. J Clin Microbiol déc. 2012;50(12):4180–2.
Thurneysen C, Boggian K. Legionella pneumophila serogroup 1 septic arthritis with probable endocarditis in an immunodeficient patient. J Clin Rheumatol août. 2014;20(5):297–8.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
EM and MR participated to conception of the work; acquisition, analysis, and interpretation of data, drafted the work and substantively revised it. EM and MR to have approved the submitted version (and any substantially modified version that involves the author's contribution to the study); EM and MR agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Roussotte, M., Massy, E. Case report of arthritis caused by Legionella anisa and review of the literature. BMC Infect Dis 22, 633 (2022). https://doi.org/10.1186/s12879-022-07475-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07475-3