Abstract
Background
Ascaris lumbricoides and Ascaris suum are the most common soil-transmitted helminths of humans and pigs, respectively. The zoonotic potential of A. suum has been a matter of debate for decades. This study was aimed to present a case of human ascariasis caused by A. suum in southern Italy.
Case presentation
A 75-year-old man presented to the department of surgery in Avellino (southern Italy) complaining of abdominal pain and vomiting. Physical examination revealed bloating and abdominal tenderness. A computed tomography scan showed air-fluid levels and small bowel distension. During exploratory laparotomy a small bowel volvulus with mesenteritis was evident and surprisingly an intraluminal worm was detected. The worm was removed with a small enterotomy and identified as an adult female of A. suum based on morphological and molecular analysis. Faecal examination revealed the presence of unfertilized Ascaris eggs with an intensity of 16 eggs per gram (EPG) of faeces. The patient was treated with mebendanzole 100 mg twice a day for 3 days. The post-operative course was regular with re-alimentation after 3 days and discharge after 12 days.
Conclusions
This report shows as A. suum can function as a relevant agent of human zoonosis. Therefore, in patients with bowel obstruction with no evident aetiology a helminthic infestation should be considered for an accurate diagnosis, especially in patients living in rural areas.
Similar content being viewed by others
Background
Ascaris lumbricoides (Linnaeus, 1758) and Ascaris suum (Goeze, 1782) are the most common soil-transmitted helminths (STHs) of humans and pigs, respectively.
Human ascariasis, caused by A. lumbricoides, is one of the neglected tropical diseases (NTDs) of greatest public health and socio-economic importance. It was estimated around 447 million people were infected in 2017 and its global burden contributes over 860,000 Disability-Adjusted Life Years (DALYs) [1]. Ascaris suum is one of the most common parasites in pigs, leading to huge economic losses in the pig industry linked mainly to reduced growth, poor feed conversion efficiency and costs of control in farms [2].
Ascaris lumbricoides and A. suum produce cross-infections between pigs and humans being species potentially zoonotic [3]. The eggs of both species are morphologically indistinguishable whereas the adults differ only in the shape of the lips and teeth measure detectable by electron microscope [4]. Due to their morphological similarities, there is currently an active debate if A. lumbricoides and A. suum are the same species [5] or whether they are different species as indicated by genetic evidence [6].
Ascariasis is generally asymptomatic both in infected humans and in pigs, however, can cause intestinal obstruction and perforation peritonitis when high parasitic loads are present. Furthermore, as Ascaris larvae develop, different stage-specific antigens are observed and various tissues are invaded, therefore the effects of infection differ over the course of larval migration and development [7]. As regards A. lumbricoides, chronic infections may also be associated with growth failure and cognitive delay in children [8].
Ascaris infection is transmitted via faecal contamination of the environment with parasite eggs. Because of this, human ascariasis is prevalent in developing countries where poor access to adequate sources of water, sanitation and hygiene, favour the transmission human-to-human [3]. Nevertheless, cases have been reported in Denmark [9], the Netherlands [10], United Kingdom [11], Austria [12], Japan [13], the United States of America [14] with the zoonotic potential of pig-derived Ascaris as a plausible source of human infection in developed countries. Cavallero et al. [15] confirmed pig as a source of human infections in Italy, and the zoonotic transmission was also reported in north-western Italy by Dutto and Petrosillo [16] that found a positive case of a hybrid genotype of A. suum/lumbricoides in a pig farmer. Despite the findings, the zoonotic potential is underestimated in these countries [12] and prevention measures have been seldom applied simultaneously in both humans and pigs. The present study reports the case of a 75-year-old male from southern Italy infected with A. suum who developed an adult intestinal worm.
Case presentation
In November 2020, a 75-year-old male with low comorbidity (hypertension, no previous abdominal surgery) was admitted to the Department of General Surgery, Frieri-Criscuoli Hospital (Avellino, Italy) with abdominal pain, vomiting and bowels not opened in the last 3 days. The patient lived in a rural area of the Campania region, southern Italy. The anamnesis revealed that until 3 years ago he had an orchard and raised chickens and pigs for his own consumption. Physical examination revealed abdominal pain with bloating. Examination of blood showed a white blood cell (WBC) count of 19.16 × 103/μL with 86.8% neutrophils and 2.9% eosinophils. Computed tomography (CT) scan exposed multiple air-fluid levels in the epigastrium and left hypochondrium with some fluid in mesenteric recesses and signs of bowel obstruction (Fig. 1). The day after the hospitalization, the symptoms persisted so the patient was referred to surgery. The explorative laparotomy revealed bowel dilatation with vascular suffering and kinking of a tract of ileus in correspondence of a severe mesenteric inflammation. During the bowel palpation, from the Treitz ligament to the ileocecal valve, a long worm-like foreign body was felt and this occasioned to a little enterotomy to extract the worm (Fig. 2). The palpation of the entire bowel did not show the presence of other ones. The worm recovered was washed in saline solution and stored in 70% (v/v) ethanol. A stool sample was also collected from the bowel of the patient and preserved at + 4 °C.
The worm and the stool samples were sent, within 24 h, to the laboratories of the WHO Collaborating Centre for diagnosis of intestinal helminths and protozoa (WHO CCITA-116, University of Naples Federico II, Italy) for further analysis.
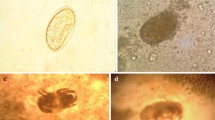
The worm was cylindrical in shape, pinkish in colour and measured 20 cm in length and 6 mm in diameter (Fig. 3). Under the stereomicroscope the helminth presented a buccal orifice with three lips (two subventral and one dorsal) and a straight posterior end (Fig. 4). The worm was identified as an adult female of Ascaris spp. [17].
A portion of the adult worm was sent in 70% (v/v) ethanol to the Department of Public Health and Infectious Diseases, Section of Parasitology (Sapienza University of Rome, Italy) for the genetic characterization.
DNA extraction was performed using the Isolate II Genomic DNA kit (Bioline, UK), according to the manufacturer’s protocol. The entire ITS nuclear region (ITS1, 5.8S, ITS2) was amplified using 20 ng of genomic DNA, 10 mM Tris–HCl (pH 8.3), 1.5 mM MgCl2 (Bioline), 40 mM of a nucleotide mix (Bioline), 20 pmol/µl each of the forward primer NC5 (59-GTAGGTGAACCTGCGGAAGGATCAT- 39) and the reverse primer NC2 (59-TTAGTTTCTTCCTCCGCT-39) described by Zhu et al. [18] and 1.0 U of BIOTAQ DNA Polymerase (Bioline) in a final volume of 50 µl. The PCR was performed in a GenePro Eurocycler Dual Block (Bioer) under the following conditions: 10 min at 95 °C (initial denaturation), 30 cycles of 30 s at 95 °C (denaturation), 40 s at 52 °C (annealing) and 75 s at 72 °C (extension), and a final elongation step of 7 min at 72 °C. A negative control (PCR master mix without genomic DNA) was included in the amplification reaction.
A PCR product of around 1000 bp was obtained. The ITS amplicon was digested with the restriction endonuclease HaeIII, as the resulting patterns have been previously proved useful for the identification of Ascaris species. This approach yielded the specific banding pattern peculiar to the A. suum genotype, showing three bands of about 610 bp, 230 bp and 140 bp (Fig. 5).
The stool sample was analysed with the FLOTAC dual technique [19] as described in Gualdieri et al. [20]. The analytic sensitivity was four eggs per gram (EPG) of faeces. Faecal examination revealed the presence of unfertilized Ascaris eggs (Fig. 6) with an intensity of 16 EPG.
The patient was treated with mebendazole 100 mg twice a day for 3 days. The stool examination at 7-days follow-up was negative. The post-operative hospitalization was regular with re-alimentation after 3 days and discharge after 12 days, asymptomatic. A week after the discharge another parasitological stool examination was negative.
Discussion and conclusions
This report shows as A. suum can function as a relevant agent of human zoonosis in non-endemic areas. Human ascariasis is generally common in developing countries where access to personal hygiene and proper sanitation practices are not available; however, increased travel and migration have made this infection more common also in non-endemic areas [8]. Furthermore, sporadic cases of human ascariasis have been reported in developed countries in North America and Europe which have been mainly associated to A. suum [21], confirming that cross-transmission of A. lumbricoides and A. suum between humans and pigs likely occurred [15]. This is particularly likely in areas where humans and pigs live in close proximity, as demonstrated by Criscione et al. [22] that identified 4% and 7% of hybrids in adult samples of Ascaris collected in Guatemala and China, respectively. Therefore, under certain conditions in which people have frequent contact with pigs and soil, possible cross-infection with Ascaris should be considered for an accurate diagnosis.
Ascaris suum is widespread and high prevalence values have been reported in pigs in western countries like Denmark (25–88%) and Canada (18–82%) [23]. In Italy, A. suum infection is widely distributed in domestic pigs [24, 25]. In southern Italy, prevalence values of 20.1% and 88% have been reported in pigs (Cringoli et al., personal communication) and wild swine [26], respectively.
In the present study, A. suum was detected in a patient who had recently raised pigs in a rural area of southern Italy. Certain farm practices might have contributed to his exposure to Ascaris eggs, such as use of pig manure as fertilizer, use of pig bedding for compost, and location of pig pens near where produce is grown.
The definitive diagnosis of ascariasis was achieved only during the surgery through the bowel palpation from the Treitz ligament to the ileocecal valve because the clinical manifestations of the patient were very heterogeneous (e.g. abdominal pain, nausea, vomiting, etc.) and not specific for a helminth infection. Furthermore, it should be emphasized that bowel volvulus could be caused by other several factors such as intestinal malrotation (mostly in children), intestinal adhesions, decreased pelvic space due to pregnancy or pelvic mass, Hirschsprung or Chagas disease. For these reasons, diagnosis of infections by Ascaris with clinical symptoms, hematological investigations, and biochemical profile is usually inconclusive [27] due to the non-specific findings. Frequent symptoms reported are abdominal pain, nausea, vomiting, diarrhoea, and presence of worms in vomit or faeces [27,28,29]. During the physical examination, abdominal tenderness, bloating, abdominal mass, or rigidity could be presents whereas x-rays could reveal air fluid levels and shadow of roundworms in some cases [28]. Finally, laboratory findings associated to ascariasis are elevated leukocytosis, eosinophilia and elevated C-reactive protein [30]. An accurate diagnosis can be made by finding the eggs or adult worms in the stool. It is important to highlight that parasitological stool examination is not a routine test and that a low parasitic load cannot be detected if the technique used is not highly sensitive.
The finding of a single worm and the low parasitic intensity (16 EPG) diagnosed using the FLOTAC technique confirmed that zoonotic cases developed a smaller number of adult worms in the intestine when infected with eggs from pig Ascaris [9, 16], making the diagnosis even more difficult.
In this case report, the bowel obstruction was not caused by worm tangle but by a localized mesenteritis with bowel kinking. In patients with bowel obstruction and with a no evident aetiology a complete bowel palpation during surgery and a pre or post-operative parasitological examination should be performed.
Because of the economic and health importance of Ascaris infection, an integrated One Health approach based on farm practices, animal husbandry and health education efforts should be addressed to reduce its transmission [14].
Prevention measures in farms should include the use of equipment for handling animal waste and stall cleaning, a proper personal hygiene (wash hands before and after contact with pigs, pig waste, or soil contaminated with pig waste) and keeping pig pens separate from vegetable fields and avoiding use of pig manure as fertilizer [14]. Furthermore, since ascariasis in pigs is frequently sub-clinical, diagnostic techniques should be used routinely (e.g. ELISA, post-mortem examination of liver and lung lesions, faecal egg count) to estimate parasite presence in farms [31, 32]. Finally, because Ascaris eggs can remain viable for extended periods in soil, it should be recommended to wash thoroughly raw produce before consumption. Furthermore, preventive measures should be included in health education programmes to reduce the risk of infection.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- STHs:
-
Soil-transmitted helminths
- NTDs:
-
Neglected tropical diseases
- DALYs:
-
Disability-Adjusted Life Years
- WBC:
-
White blood cell
- CT:
-
Computed tomography
- EPG:
-
Eggs per gram
References
Easton A, Gao S, Lawton SP, Bennuru S, Khan A, Dahlstrom E, Oliveira RG, Kepha S, Porcella SF, Webster J, Anderson R, Grigg ME, Davis RE, Wang J, Nutman TB. Molecular evidence of hybridization between pig and human Ascaris indicates an interbred species complex infecting humans. eLife. 2020;9:e61562.
Holland C. Ascaris: the neglected parasite. London: Academic Press; 2013.
Betson M, Nejsum P, Bendall RP, Deb RM, Stothard JR. Molecular epidemiology of ascariasis: a global perspective on the transmission dynamics of Ascaris in people and pigs. J Infect Dis. 2014;210(6):932–41.
Monteiro KJL, Calegar DA, Santos JP, Bacelar PAA, Coronato-Nunes B, Reis ERC, Boia MN, Carvalho-Costa FA, Jaeger LH. Genetic diversity of Ascaris spp. infecting humans and pigs in distinct Brazilian regions, as revealed by mitochondrial DNA. PLoS ONE. 2019;14(6):e0218867.
Leles D, Gardner SL, Reinhard K, Iñiguez A, Araujo A. Are Ascaris lumbricoides and Ascaris suum a single species? Parasit Vectors. 2012;5:42.
Zhou C, Chen J, Niu H, Ouyang S, Wu X. Study on the population evolution of Ascaris lumbricoides and Ascaris suum based on whole genome resequencing. Vet Parasitol. 2020;279:109062.
Dold C, Holland CV. Ascaris and ascariasis. Microbes Infect. 2011;13(7):632–7.
Lamberton PH, Jourdan PM. Human ascariasis: diagnostics update. Curr Trop Med Rep. 2015;2(4):189–200.
Nejsum P, Parker ED Jr, Frydenberg J, Roepstorff A, Boes J, Haque R, Astrup I, Prag J, Skov Sørensen UB. Ascariasis is a zoonosis in Denmark. J Clin Microbiol. 2005;43(3):1142–8.
Pinelli E, Herremans T, Harms MG, Hoek D, Kortbeek LM. Toxocara and Ascaris seropositivity among patients suspected of visceral and ocular larva migrans in the Netherlands: trends from 1998 to 2009. Eur J Clin Microbiol Infect Dis. 2011;30(7):873–9.
Bendall RP, Barlow M, Betson M, Stothard JR, Nejsum P. Zoonotic ascariasis, United Kingdom. Emerg Infect Dis. 2011;17(10):1964–6.
Schneider R, Auer H. Incidence of Ascaris suum-specific antibodies in Austrian patients with suspected larva migrans visceralis (VLM) syndrome. Parasitol Res. 2016;115(3):1213–9.
Arizono N, Yoshimura Y, Tohzaka N, Yamada M, Tegoshi T, Onishi K, Uchikawa R. Ascariasis in Japan: is pig-derived Ascaris infecting humans? Jpn J Infect Dis. 2010;63(6):447–8.
Miller LA, Colby K, Manning SE, Hoenig D, McEvoy E, Montgomery S, Mathison B, de Almeida M, Bishop H, Dasilva A, Sears S. Ascariasis in humans and pigs on small-scale farms, Maine, USA, 2010–2013. Emerg Infect Dis. 2015;21(2):332–4.
Cavallero S, Snabel V, Pacella F, Perrone V, D'Amelio S. Phylogeographical studies of Ascaris spp. based on ribosomal and mitochondrial DNA sequences. PLoS Negl Trop Dis. 2013;7(4):e2170.
Dutto M, Petrosillo N. Hybrid Ascaris suum/lumbricoides (ascarididae) infestation in a pig farmer: a rare case of zoonotic ascariasis. Cent Eur J Public Health. 2013;21(4):224–6.
Fagerholm HP, Nansen P, Roepstorff A, Frandsen F, Eriksen L. Growth and structural features of the adult stage of Ascaris suum (Nematoda, Ascaridoidea) from experimentally infected domestic pigs. J Parasitol. 1998;84(2):269–77.
Zhu X, Chilton NB, Jacobs DE, Boes J, Gasser RB. Characterisation of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. Int J Parasitol. 1999;29(3):469–78.
Cringoli G, Rinaldi L, Maurelli MP, Utzinger J. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc. 2010;5(3):503–15.
Gualdieri L, Piemonte M, Alfano S, Maffei R, Della Pepa ME, Rinaldi L, Galdiero M, Galdiero M, Cringoli G. Immigrants living in an urban milieu with sanitation in Southern Italy: persistence and transmission of intestinal parasites. Parasitol Res. 2016;115(3):1315–23.
Peng W, Yuan K, Zhou X, Hu M, Abs EL-Osta YG, Gasser RB. Molecular epidemiological investigation of Ascaris genotypes in China based on single-strand conformation polymorphism analysis of ribosomal DNA. Electrophoresis. 2003;24(14):2308–15.
Criscione CD, Anderson JD, Sudimack D, Peng W, Jha B, Williams-Blangero S, Anderson TJ. Disentangling hybridization and host colonization in parasitic roundworms of humans and pigs. Proc Biol Sci. 2007;274(1626):2669–77.
Martínez-Pérez JM, Vandekerckhove E, Vlaminck J, Geldhof P, Martínez-Valladares M. Serological detection of Ascaris suum at fattening pig farms is linked with performance and management indices. Vet Parasitol. 2017;248:33–8.
Ceccarelli M, Leprini E, Sechi P, Iulietto MF, Grispoldi L, Goretti E, Cenci-Goga BT. Analysis of the causes of the seizure and destruction of carcasses and organs in a slaughterhouse in central Italy in the 2010–2016 period. Ital J Food Saf. 2018;7(1):6899.
Guardone L, Vitali A, Fratini F, Pardini S, Cenci Goga BT, Nucera D, Armani A. A retrospective study after 10 years (2010–2019) of meat inspection activity in a domestic swine abattoir in Tuscany: the slaughterhouse as an epidemiological observatory. Animals (Basel). 2020;10(10):1907.
Castagna F, Musella V, Esposito L, Poerio A, Rinaldi L, Bosco A, Cringoli G, Britti D. Helminths of wild boar (Sus scrofa) in the Calabrian region of southern Italy. J Wildl Dis. 2019;55(2):416–20.
Khan MW, Ghauri SK. Small bowel Ascaris infestation: a diagnostic challenge. Int J Gen Med. 2016;9:99–101.
Andrade AM, Perez Y, Lopez C, Collazos SS, Andrade AM, Ramirez GO, Andrade LM. Intestinal obstruction in a 3-year-old girl by Ascaris lumbricoides infestation: case report and review of the literature. Medicine (Baltimore). 2015;94(16):e655.
Mbanga CM, Ombaku KS, Fai KN, Agbor VN. Small bowel obstruction complicating an Ascaris lumbricoides infestation in a 4-year-old male: a case report. J Med Case Rep. 2019;13(1):155.
Claus PE, Ceuppens AS, Cool M, Alliet G. Ascaris lumbricoides: challenges in diagnosis, treatment and prevention strategies in a European refugee camp. Acta Clin Belg. 2018;73(6):431–4.
Vlaminck J, Levecke B, Vercruysse J, Geldhof P. Advances in the diagnosis of Ascaris suum infections in pigs and their possible applications in humans. Parasitology. 2014;141(14):1904–11.
Vlaminck J, Düsseldorf S, Heres L, Geldhof P. Serological examination of fattening pigs reveals associations between Ascaris suum, lung pathogens and technical performance parameters. Vet Parasitol. 2015;210(3–4):151–8.
Acknowledgements
Not applicable.
Funding
No funding was required for this case report.
Author information
Authors and Affiliations
Contributions
GR, LDL and GG were involved in case identification, clinical examinations, worm extraction and writing of the manuscript. PP and PC performed morphological identification of the worm, coprological analysis and helped to draft and editing of the manuscript. GG, GC and LR planned, reviewed and approved the final manuscript. SC and SDA coordinated molecular diagnostic techniques, analysis of data and writing of the manuscript. All authors critically reviewed the manuscript for publication. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Romano, G., Pepe, P., Cavallero, S. et al. Ascariasis in a 75-year-old man with small bowel volvulus: a case report. BMC Infect Dis 21, 1045 (2021). https://doi.org/10.1186/s12879-021-06718-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06718-z