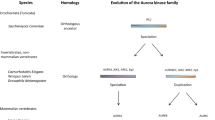
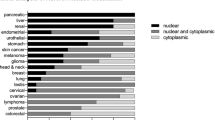
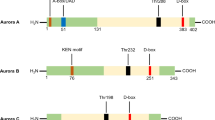
Abstract
Aurora kinases are essential players in mammalian cell division. These kinases are involved in the regulation of spindle dynamics, microtubule–kinetochore interactions, and chromosome condensation and orientation during mitosis. At least three members of the Aurora family – Aurora kinases A, B, and C – have been identified in mammals. Aurora B is essential for maintaining genomic stability and normal cell division. Mutations and dysregulation of this kinase are implicated in tumor initiation and progression. In this review, we discuss the functions of Aurora B, the relationship between increased Aurora B activity and carcinogenesis, and the prospects for the use of Aurora B kinase inhibitors in antitumor therapy.


Similar content being viewed by others
Abbreviations
- CPC:
-
chromosomal passenger complex
- INCENP:
-
inner centromere protein
- SAC:
-
spindle assembly checkpoint
References
Bischoff, J. R., Anderson, L., Zhu, Y., Mossie, K., Ng, L., Souza, B., Schryver, B., Flanagan, P., Clairvoyant, F., Ginther, C., Chan, C. S., Novotny, M., Slamon, D. J., and Plowman, G. D. (1998) A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers, EMBO J., 17, 3052-3065, https://doi.org/10.1093/EMBOJ/17.11.3052.
Francisco, L., Wang, W., and Chan, C. S. (1994) Type 1 protein phosphatase acts in opposition to IpL1 protein kinase in regulating yeast chromosome segregation, Mol. Cell. Biol., 14, 4731-4740, https://doi.org/10.1128/MCB.14.7.4731-4740.1994.
Glover, D. M., Leibowitz, M. H., McLean, D. A., and Parry, H. (1995) Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles, Cell, 81, 95-105, https://doi.org/10.1016/0092-8674(95)90374-7.
Fu, J., Bian, M., Jiang, Q., and Zhang, C. (2007) Roles of Aurora kinases in mitosis and tumorigenesis, Mol. Cancer Res., 5, 1-10, https://doi.org/10.1158/1541-7786.MCR-06-0208.
Sugimoto, K., Urano, T., Zushi, H., Inoue, K., Tasaka, H., Tachibana, M., and Dotsu, M. (2002) Molecular dynamics of Aurora-A kinase in living mitotic cells simultaneously visualized with histone H3 and nuclear membrane protein importinalpha, Cell Structure Funct., 27, 457-467, https://doi.org/10.1247/CSF.27.457.
Carmena, M., Wheelock, M., Funabiki, H., and Earnshaw, W. C. (2012) The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis, Nat. Rev. Mol. Cell Biol., 13, 789-803, https://doi.org/10.1038/NRM3474.
Broad, A. J., and DeLuca, J. G. (2020) The right place at the right time: Aurora B kinase localization to centromeres and kinetochores, Essays Biochem., 64, 299-311, https://doi.org/10.1042/EBC20190081.
Ahmed, A., Shamsi, A., Mohammad, T., Hasan, G. M., Islam, A., and Hassan, M. I. (2021) Aurora B kinase: a potential drug target for cancer therapy, J. Cancer Res. Clin. Oncol., 147, 2187-2198, https://doi.org/10.1007/S00432-021-03669-5.
Kimura, M., Matsuda, Y., Yoshioka, T., and Okano, Y. (1999) Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3, J. Biol. Chem., 274, 7334-7340, https://doi.org/10.1074/JBC.274.11.7334.
Sasai, K., Katayama, H., Hawke, D. H., and Sen, S. (2016) Aurora-C interactions with survivin and INCENP reveal shared and distinct features compared with Aurora-B chromosome passenger protein complex, PLoS One, 11, e0157305, https://doi.org/10.1371/JOURNAL.PONE.0157305.
Vader, G., Medema, R. H., and Lens, S. M. A. (2006) The chromosomal passenger complex: guiding Aurora-B through mitosis, J. Cell Biol., 173, 833-837, https://doi.org/10.1083/JCB.200604032.
Hindriksen, S., Lens, S. M. A., and Hadders, M. A. (2017) The ins and outs of Aurora B inner centromere localization, Front. Cell Dev. Biol., 5, 112, https://doi.org/10.3389/FCELL.2017.00112.
Bishop, J. D., and Schuniacher, J. M. (2002) Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity, J. Biol. Chem., 277, 27577-27580, https://doi.org/10.1074/JBC.C200307200.
Honda, R., Körner, R., and Nigg, E. A. (2003) Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis, Mol. Biol. Cell, 14, 3325-3341, https://doi.org/10.1091/MBC.E02-11-0769.
McVey, S. L., Cosby, J. K., and Nannas, N. J. (2021) Aurora B tension sensing mechanisms in the kinetochore ensure accurate chromosome segregation, Int. J. Mol. Sci., 22, 8818, https://doi.org/10.3390/IJMS22168818.
Krenn, V., and Musacchio, A. (2015) The Aurora B kinase in chromosome bi-orientation and spindle checkpoint signaling, Front. Oncol., 5, 225, https://doi.org/10.3389/FONC.2015.00225.
Van der Horst, A., Vromans, M. J. M., Bouwman, K., van der Waal, M. S., Hadders, M. A., and Lens, S. M. A. (2015) Inter-domain cooperation in INCENP promotes Aurora B relocation from centromeres to microtubules, Cell Rep., 12, 380-387, https://doi.org/10.1016/J.CELREP.2015.06.038.
Caldas, G. V., DeLuca, K. F., and DeLuca, J. G. (2013) KNL1 facilitates phosphorylation of outer kinetochore proteins by promoting Aurora B kinase activity, J. Cell Biol., 203, 957-969, https://doi.org/10.1083/JCB.201306054.
Hadders, M. A., Hindriksen, S., Truong, M. A., Mhaskar, A. N., Pepijn Wopken, J., Vromans, M. J. M., and Lens, S. M. A. (2020) Untangling the contribution of Haspin and Bub1 to Aurora B function during mitosis, J. Cell Biol., 219, e201907087, https://doi.org/10.1083/JCB.201907087.
Murata-Hori, M., Tatsuka, M., and Wang, Y. L. (2002) Probing the dynamics and functions of aurora B kinase in living cells during mitosis and cytokinesis, Mol. Biol. Cell, 13, 1099-1108, https://doi.org/10.1091/MBC.01-09-0467.
Titova, E., Shagieva, G., Ivanova, O., Domnina, L., Domninskaya, M., Strelkova, O., Khromova, N., Kopnin, P., Chernyak, B., Skulachev, V., and Dugina, V. (2018) Mitochondria-targeted antioxidant SkQ1 suppresses fibrosarcoma and rhabdomyosarcoma tumour cell growth, Cell Cycle, 17, 1797-1811, https://doi.org/10.1080/15384101.2018.1496748.
Duro, E., and Marston, A. L. (2015) From equator to pole: splitting chromosomes in mitosis and meiosis, Genes Dev., 29, 109-122, https://doi.org/10.1101/GAD.255554.114.
Foley, E. A., and Kapoor, T. M. (2013) Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore, Nat. Rev. Mol. Cell Biol., 14, 25-37, https://doi.org/10.1038/NRM3494.
Nezi, L., and Musacchio, A. (2009) Sister chromatid tension and the spindle assembly checkpoint, Curr. Opin. Cell Biol., 21, 785-795, https://doi.org/10.1016/J.CEB.2009.09.007.
Hauf, S., Cole, R. W., LaTerra, S., Zimmer, C., Schnapp, G., Walter, R., Heckel, A., van Meel, J., Rieder, C. L., and Peters, J.-M. (2003) The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint, J. Cell Biol., 161, 281-294, https://doi.org/10.1083/jcb.200208092.
Sen, O., Harrison, J. U., Burroughs, N. J., and McAinsh, A. D. (2021) Kinetochore life histories reveal an Aurora-B-dependent error correction mechanism in anaphase, Dev. Cell, 56, 3082, https://doi.org/10.1016/J.DEVCEL.2021.10.007.
Rieder, C. L., Cole, R. W., Khodjakov, A., and Sluder, G. (1995) The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores, J. Cell Biol., 130, 941-948, https://doi.org/10.1083/JCB.130.4.941.
DeLuca, J. G., Gall, W. E., Ciferri, C., Cimini, D., Musacchio, A., and Salmon, E. D. (2006) Kinetochore microtubule dynamics and attachment stability are regulated by Hec1, Cell, 127, 969-982, https://doi.org/10.1016/J.CELL.2006.09.047.
DeLuca, J. G., and Musacchio, A. (2012) Structural organization of the kinetochore-microtubule interface, Curr. Opin. Cell Biol., 24, 48-56, https://doi.org/10.1016/J.CEB.2011.11.003.
DeLuca, K. F., Lens, S. M. A., and DeLuca, J. G. (2011) Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis, J. Cell Sci., 124, 622-634, https://doi.org/10.1242/JCS.072629.
Yoo, T. Y., Choi, J. M., Conway, W., Yu, C. H., Pappu, R. V., and Needleman, D. J. (2018) Measuring NDC80 binding reveals the molecular basis of tension-dependent kinetochore-microtubule attachments, eLife, 7, e36392, https://doi.org/10.7554/ELIFE.36392.
Etemad, B., Kuijt, T. E. F., and Kops, G. J. P. L. (2015) Kinetochore-microtubule attachment is sufficient to satisfy the human spindle assembly checkpoint, Nat. Commun., 6, 8987, https://doi.org/10.1038/NCOMMS9987.
Musacchio, A., and Salmon, E. D. (2007) The spindle-assembly checkpoint in space and time, Nat. Rev. Mol. Cell Biol., 8, 379-393, https://doi.org/10.1038/NRM2163.
Mierzwa, B., and Gerlich, D. W. (2014) Cytokinetic abscission: molecular mechanisms and temporal control, Dev. Cell, 31, 525-538, https://doi.org/10.1016/J.DEVCEL.2014.11.006.
Gisselsson, D. (2008) Classification of chromosome segregation errors in cancer, Chromosoma, 117, 511-519, https://doi.org/10.1007/S00412-008-0169-1.
Petsalaki, E., and Zachos, G. (2016) Clks 1, 2 and 4 prevent chromatin breakage by regulating the Aurora B-dependent abscission checkpoint, Nat. Commun., 7, 11451, https://doi.org/10.1038/NCOMMS11451.
Goto, H., Yasui, Y., Kawajiri, A., Nigg, E. A., Terada, Y., Tatsuka, M., Nagata, K., and Inagaki, M. (2003) Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process, J. Biol. Chem., 278, 8526-8530, https://doi.org/10.1074/jbc.M210892200.
Christ, L., Raiborg, C., Wenzel, E. M., Campsteijn, C., and Stenmark, H. (2017) Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery, Trends Biochem. Sci., 42, 42-56, https://doi.org/10.1016/J.TIBS.2016.08.016.
Borah, N. A., and Reddy, M. M. (2021) Aurora kinase B inhibition: a potential therapeutic strategy for cancer, Molecules, 26, 1981, https://doi.org/10.3390/MOLECULES26071981.
González-Loyola, A., Fernández-Miranda, G., Trakala, M., Partida, D., Samejima, K., Ogawa, H., Cañamero, M., de Martino, A., Martínez-Ramírez, Á., de Cárcer, G., Pérez de Castro, I., Earnshaw, W. C., and Malumbres, M. (2015) Aurora B overexpression causes aneuploidy and p21Cip1 repression during tumor development, Mol. Cell. Biol., 35, 3566-3578, https://doi.org/10.1128/MCB.01286-14.
Smith, S. L., Bowers, N. L., Betticher, D. C., Gautschi, O., Ratschiller, D., Hoban, P. R., Booton, R., Santibáñez-Koref, M. F., and Heighway, J. (2005) Overexpression of aurora B kinase (AURKB) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability, Br. J. Cancer, 93, 719-729, https://doi.org/10.1038/sj.bjc.6602779.
Vischioni, B., Oudejans, J. J., Vos, W., Rodriguez, J. A., and Giaccone, G. (2006) Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients, Mol. Cancer Ther., 5, 2905-2913, https://doi.org/10.1158/1535-7163.MCT-06-0301.
Sorrentino, R., Libertini, S., Pallante, P. L., Troncone, G., Palombini, L., Bavetsias, V., Spalletti-Cernia, D., Laccetti, P., Linardopoulos, S., Chieffi, P., Fusco, A., and Portella, G. (2005) Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation, J. Clin. Endocrinol. Metab., 90, 928-935, https://doi.org/10.1210/JC.2004-1518.
Huang, D., Huang, Y., Huang, Z., Weng, J., Zhang, S., and Gu, W. (2019) Relation of AURKB over-expression to low survival rate in BCRA and reversine-modulated aurora B kinase in breast cancer cell lines, Cancer Cell Int., 19, 166, https://doi.org/10.1186/S12935-019-0885-Z.
Chieffi, P., Cozzolino, L., Kisslinger, A., Libertini, S., Staibano, S., Mansueto, G., De Rosa, G., Villacci, A., Vitale, M., Linardopoulos, S., Portella, G., and Tramontano, D. (2006) Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation, Prostate, 66, 326-333, https://doi.org/10.1002/PROS.20345.
Pohl, A., Azuma, M., Zhang, W., Yang, D., Ning, Y., Winder, T., Danenberg, K., and Lenz, H. J. (2011) Pharmacogenetic profiling of Aurora kinase B is associated with overall survival in metastatic colorectal cancer, Pharmacogenom. J., 11, 93-99, https://doi.org/10.1038/TPJ.2010.18.
Chang, X., Zhang, T., Wang, Q., Rathore, M. G., Reddy, K., Chen, H., Shin, S. H., Ma, W. Y., Bode, A. M., and Dong, Z. (2020) HI-511 overcomes melanoma drug resistance via targeting AURKB and BRAF V600E, Theranostics, 10, 9721-9740, https://doi.org/10.7150/THNO.44342.
Failes, T. W., Mitic, G., Abdel-Halim, H., Po’uha, S. T., Liu, M., Hibbs, D. E., and Kavallaris, M. (2012) Evolution of resistance to Aurora kinase B inhibitors in leukaemia cells, PLoS One, 7, e30734, https://doi.org/10.1371/JOURNAL.PONE.0030734.
Skaland, I., Janssen, E. A. M., Gudlaugsson, E., Hui Ru Guo, L., and Baak, J. P. A. (2009) The prognostic value of the proliferation marker Phosphohistone H3 (PPH3) in luminal, basal-like and triple negative phenotype invasive lymph node-negative breast cancer, Cell. Oncol., 31, 261-271, https://doi.org/10.3233/CLO-2009-0464.
Den Hollander, J., Rimpi, S., Doherty, J. R., Rudelius, M., Buck, A., Hoellein, A., Kremer, M., Graf, N., Scheerer, M., Hall, M. A., Goga, A., von Bubnoff, N., Duyster, J., Peschel, C., Cleveland, J. L., Nilsson, J. A., and Keller, U. (2010) Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state, Blood, 116, 1498-1505, https://doi.org/10.1182/BLOOD-2009-11-251074.
Borah, N. A., Sradhanjali, S., Barik, M. R., Jha, A., Tripathy, D., Kaliki, S., Rath, S., Raghav, S. K., Patnaik, S., Mittal, R., and Reddy, M. M. (2021) Aurora kinase B expression, its regulation and therapeutic targeting in human retinoblastoma, Invest. Ophthalmol. Vis. Sci., 62, 16, https://doi.org/10.1167/IOVS.62.3.16.
Bogen, D., Wei, J. S., Azorsa, D. O., Ormanoglu, P., Buehler, E., Guha, R., Keller, J. M., Mathews Griner, L. A., Ferrer, M., Song, Y. K., Liao, H., Mendoza, A., Gryder, B. E., Sindri, S., He, J., Wen, X., Zhang, S., Shern, J. F., Yohe, M. E., Taschner-Mandl, S., Shohet, J. M., Thomas, C. J., Martin, S. E., Ambros, P. F., and Khan, J. (2015) Aurora B kinase is a potent and selective target in MYCN-driven neuroblastoma, Oncotarget, 6, 35247-35262, https://doi.org/10.18632/ONCOTARGET.6208.
Teng, C.L., Hsieh, Y. C., Phan, L., Shin, J., Gully, C., Velazquez-Torres, G., Skerl, S., Yeung, S. C. J., Hsu, S. L., and Lee, M. H. (2012) FBXW7 is involved in Aurora B degradation, Cell Cycle, 11, 4059-4068, https://doi.org/10.4161/CC.22381.
Gully, C. P., Velazquez-Torres, G., Shin, J. H., Fuentes-Mattei, E., Wang, E., Carlock, C., Chen, J., Rothenberg, D., Adams, H. P., Choi, H. H., Guma, S., Phan, L., Chou, P. C., Su, C. H., Zhang, F., Chen, J. S., Yang, T. Y., Yeung, S. C. J., and Lee, M. H. (2012) Aurora B kinase phosphorylates and instigates degradation of p53, Proc. Natl. Acad. Sci. USA, 109, E1513-E1522, https://doi.org/10.1073/PNAS.1110287109.
Kanagasabai, T., Venkatesan, T., Natarajan, U., Alobid, S., Alhazzani, K., Algahtani, M., and Rathinavelu, A. (2020) Regulation of cell cycle by MDM2 in prostate cancer cells through Aurora Kinase-B and p21WAF1/CIP1 mediated pathways, Cell. Signal., 66, 109435, https://doi.org/10.1016/J.CELLSIG.2019.109435.
Schecher, S., Walter, B., Falkenstein, M., Macher-Goeppinger, S., Stenzel, P., Krümpelmann, K., Hadaschik, B., Perner, S., Kristiansen, G., Duensing, S., Roth, W., and Tagscherer, K. E. (2017) Cyclin K dependent regulation of Aurora B affects apoptosis and proliferation by induction of mitotic catastrophe in prostate cancer, Int. J. Cancer, 141, 1643-1653, https://doi.org/10.1002/IJC.30864.
Nair, J. S., Ho, A. L., Tse, A. N., Coward, J., Cheema, H., Ambrosini, G., Keen, N., Schwartz, G. K. (2009) Aurora B kinase regulates the postmitotic endoreduplication checkpoint via phosphorylation of the retinoblastoma protein at serine 780, Mol. Biol. Cell, 20, 2218-2228, https://doi.org/10.1091/MBC.E08-08-0885.
Hartsink-Segers, S. A., Zwaan, C. M., Exalto, C., Luijendijk, M. W. J., Calvert, V. S., Petricoin, E. F., Evans, W. E., Reinhardt, D., De Haas, V., Hedtjärn, M., Hansen, B. R., Koch, T., Caron, H. N., Pieters, R., and Den Boer, M. L. (2013) Aurora kinases in childhood acute leukemia: the promise of aurora B as therapeutic target, Leukemia, 27, 560, https://doi.org/10.1038/LEU.2012.256.
Kovacs, A. H., Zhao, D., and Hou, J. (2023) Aurora B inhibitors as cancer therapeutics, Molecules, 28, 3385, https://doi.org/10.3390/MOLECULES28083385.
Adams, N. D., Adams, J. L., Burgess, J. L., Chaudhari, A. M., Copeland, R. A., Donatelli, C. A., Drewry, D. H., Fisher, K. E., Hamajima, T., Hardwicke, M. A., Huffman, W. F., Koretke-Brown, K. K., Lai, Z. V., McDonald, O. B., Nakamura, H., Newlander, K. A., Oleykowski, C. A., Parrish, C. A., Patrick, D. R., Plant, R., Sarpong, M. A., Sasaki, K., Schmidt, S. J., Silva, D. J., Sutton, D., Tang, J., Thompson, C. S., Tummino, P. J., Wang, J. C., Xiang, H., Yang, J., and Dhanak, D. (2010) Discovery of GSK1070916, a potent and selective inhibitor of Aurora B/C kinase, J. Med. Chem., 53, 3973-4001, https://doi.org/10.1021/JM901870Q.
Zhou, Y., Shan, S., Li, Z. Bin., Xin, L. J., Pan, D. S., Yang, Q. J., Liu, Y. P., Yue, X. P., Liu, X. R., Gao, J. Z., Zhang, J. W., Ning, Z. Q., and Lu, X. P. (2017) CS2164, a novel multi-target inhibitor against tumor angiogenesis, mitosis and chronic inflammation with anti-tumor potency, Cancer Sci., 108, 469-477, https://doi.org/10.1111/CAS.13141.
Falchook, G. S., Bastida, C. C., and Kurzrock, R. (2015) Aurora kinase inhibitors in oncology clinical trials: current state of the progress, Semin. Oncol., 42, 832-848, https://doi.org/10.1053/J.SEMINONCOL.2015.09.022.
Boss, D. S., Witteveen, P. O., van der Sar, J., Lolkema, M. P., Voest, E. E., Stockman, P. K., Ataman, O., Wilson, D., Das, S., and Schellens, J. H. (2011) Clinical evaluation of AZD1152, an i.v. inhibitor of Aurora B kinase, in patients with solid malignant tumors, Ann. Oncol. Off. J. Eur. Soc. Med. Oncol., 22, 431-437, https://doi.org/10.1093/ANNONC/MDQ344.
Schwartz, G. K., Carvajal, R. D., Midgley, R., Rodig, S. J., Stockman, P. K., Ataman, O., Wilson, D., Das, S., Shapiro, G. I. (2013) Phase I study of barasertib (AZD1152), a selective inhibitor of Aurora B kinase, in patients with advanced solid tumors, Invest. New Drugs, 31, 370-380, https://doi.org/10.1007/S10637-012-9825-7.
Löwenberg, B., Muus, P., Ossenkoppele, G., Rousselot, P., Cahn, J.Y., Ifrah, N., Martinelli, G., Amadori, S., Berman, E., Sonneveld, P., Jongen-Lavrencic, M., Rigaudeau, S., Stockman, P., Goudie, A., Faderl, S., Jabbour, E., and Kantarjian, H. (2011) Phase 1/2 study to assess the safety, efficacy, and pharmacokinetics of barasertib (AZD1152) in patients with advanced acute myeloid leukemia, Blood, 118, 6030-6036, https://doi.org/10.1182/BLOOD-2011-07-366930.
Tsuboi, K., Yokozawa, T., Sakura, T., Watanabe, T., Fujisawa, S., Yamauchi, T., Uike, N., Ando, K., Kihara, R., Tobinai, K., Asou, H., Hotta, T., and Miyawaki, S. (2011) A Phase I study to assess the safety, pharmacokinetics and efficacy of barasertib (AZD1152), an Aurora B kinase inhibitor, in Japanese patients with advanced acute myeloid leukemia, Leuk. Res., 35, 1384-1389, https://doi.org/10.1016/J.LEUKRES.2011.04.008.
Kantarjian, H. M., Martinelli, G., Jabbour, E. J., Quintás-Cardama, A., Ando, K., Bay, J. O., Wei, A., Gröpper, S., Papayannidis, C., Owen, K., Pike, L., Schmitt, N., Stockman, P. K., and Giagounidis, A. (2013) Stage I of a phase 2 study assessing the efficacy, safety, and tolerability of barasertib (AZD1152) versus low-dose cytosine arabinoside in elderly patients with acute myeloid leukemia, Cancer, 119, 2611-2619, https://doi.org/10.1002/CNCR.28113.
Dennis, M., Davies, M., Oliver, S., D’Souza, R., Pike, L., and Stockman, P. (2012) Phase I study of the Aurora B kinase inhibitor barasertib (AZD1152) to assess the pharmacokinetics, metabolism and excretion in patients with acute myeloid leukemia, Cancer Chemother. Pharmacol., 70, 461-469, https://doi.org/10.1007/S00280-012-1939-2.
McNeish, I., Anthoney, A., Loadman, P., Berney, D., Joel, S., Halford, S. E. R., Buxton, E., Race, A., Ikram, M., Scarsbrook, A., Patikis, A., Rockall, A., Dobbs, N. A., and Twelves, C. (2013) A phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of the selective aurora kinase inhibitor GSK1070916A, J. Clin. Oncol., 31, 2525-2525, https://doi.org/10.1200/JCO.2013.31.15_SUPPL.2525.
Borthakur, G., Dombret, H., Schafhausen, P., Brummendorf, T. H., Boisse, N., Jabbour, E., Mariani, M., Capolongo, L., Carpinelli, P., Davite, C., Kantarjian, H., and Cortes, J. E. (2015) A phase I study of danusertib (PHA-739358) in adult patients with accelerated or blastic phase chronic myeloid leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia resistant or intolerant to imatinib and/or other second generation c-ABL therapy, Haematologica, 100, 898-904, https://doi.org/10.3324/HAEMATOL.2014.115279.
Wu, X., Liu, W., Cao, Q., Chen, C., Chen, Z., Xu, Z., Li, W., Liu, F., and Yao, X. (2014) Inhibition of Aurora B by CCT137690 sensitizes colorectal cells to radiotherapy, J. Exp. Clin. Cancer Res., 33, 13, https://doi.org/10.1186/1756-9966-33-13.
Tao, Y., Leteur, C., Calderaro, J., Girdler, F., Zhang, P., Frascogna, V., Varna, M., Opolon, P., Castedo, M., Bourhis, J., Kroemer, G., and Deutsch, E. (2009) The aurora B kinase inhibitor AZD1152 sensitizes cancer cells to fractionated irradiation and induces mitotic catastrophe, Cell Cycle, 8, 3172-3181, https://doi.org/10.4161/cc.8.19.9729.
Sak, A., Stuschke, M., Groneberg, M., Kübler, D., Pöttgen, C., and Eberhardt, W. E. E. (2012) Inhibiting the aurora B kinase potently suppresses repopulation during fractionated irradiation of human lung cancer cell lines, Int. J. Radiat. Oncol. Biol. Phys., 84, 492-499, https://doi.org/10.1016/J.IJROBP.2011.12.021.
Liu, N., Wang, Y. A., Sun, Y., Ecsedy, J., Sun, J., Li, X., and Wang, P. (2019) Inhibition of Aurora A enhances radiosensitivity in selected lung cancer cell lines, Respir. Res., 20, 230, https://doi.org/10.1186/S12931-019-1194-8.
Funding
The work was supported by the Russian Science Foundation (project 23-15-00433).
Author information
Authors and Affiliations
Contributions
V.D. conceived and supervised the study; E.T. and G.Sh. wrote the manuscript; G.Sh. and P.K. edited the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest. This article does not contain description of studies involving human participants or animals performed by any of the authors.
Additional information
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Titova, E., Shagieva, G., Dugina, V. et al. The Role of Aurora B Kinase in Normal and Cancer Cells. Biochemistry Moscow 88, 2054–2062 (2023). https://doi.org/10.1134/S0006297923120088
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923120088