Abstract
Potassium-ion batteries (PIBs) by virtue of their strong cost competitiveness and similar electrochemical properties to lithium-ion batteries have been deemed to be a promising electrochemical energy storage technology. To promote the application in the commercial market, developing electrode materials with high specific capacities, superior cycling stability, and reliable safety is of great importance. Anode materials as an important component of PIBs play a decisive role, among which two-dimensional transition metal chalcogenides (2D TMCs) have attracted wide attention owing to their unique material and electrochemical properties. In the 2D TMCs’ family, molybdenum chalcogenides as flagship are the most studied materials and demonstrated the potential as anodes. With the deepening of research on 2D TMCs, another shining member that possesses similar properties to molybdenum chalcogenides, tungsten chalcogenides (WS2, WSe2, and WTe2), has aroused tremendous attention. Despite many inspiring results, various challenges remain to be further addressed; meanwhile, some results are still unclear and disputed. Herein, this review first introduces their material properties and electrochemical storage mechanisms. Then, we systematically overview the research progress and put forward promoting improvement strategies. Finally, challenges and opportunities that would be future research directions are discussed.


(Reproduced with permission from Ref. [41]. Copyright 2020, Wiley)

(Reproduced with permission from Ref. [51]. Copyright 2021, The Royal Society of Chemistry)

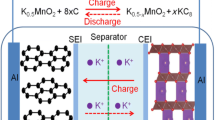
(Reproduced with permission from Ref. [51]. Copyright 2021, The Royal Society of Chemistry). d–e Transmission electron microscope and SEM images of WS2-SPAN. (Reproduced with permission from Ref. [53]. Copyright 2023, Elsevier). f Electron paramagnetic resonance spectra of WS2 (P-WS2) and WS2 with sulfur vacancies (Sv-WS2). g Rate performance of commercial WS2, P-WS2, and Sv-WS2. (Reproduced with permission from Ref. [54]. Copyright 2022, Elsevier). h Schematic of WS2 nanosheets (WS2 NS), WS2 with sulfur vacancies (Vs-WS2 NS), and selenium-filled Vs-WS2 nanosheets (Vs-WS2-Se NS). (Reproduced with permission from Ref [55]. Copyright 2022, American Chemical Society). i Cycling performance of WS2/C at 10 C. j Schematic illustration of the K+ storage mechanism in WS2/C. (Reproduced with permission from Ref. [56] Copyright 2022, Elsevier)

(Reproduced with permission from Ref. [60]. Copyright 2021, Elsevier). b Schematic illustration of the preparation of WSe2/N, P–C composite. c Long-term cycling performance at 1.0 A⋅g–1 and coulombic efficiency of WSe2/N, P–C-2. (Reproduced with permission from Ref. [59]. Copyright 2020, Elsevier). d Schematic illustration of the synthesis of WSe2@N-doped C nanorods. (Reproduced with permission from Ref. [61]. Copyright 2022, Wiley). e Schematic illustration of the synthesis of KxWSe2. f Structural evolutions of WSe2 during the “hydrothermal potassiation” process. g Long-term cycle performance of SP-KxWSe2 at 1.0 A⋅g–1. (Reproduced with permission from Ref. [62]. Copyright 2023, Wiley)

(Reproduced with permission from Ref. [63]. Copyright 2020, IOP publishing)
Similar content being viewed by others
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.
References
Balat M. Usage of energy sources and environmental problems. Energ Explor Exploit. 2005;23(2):141. https://doi.org/10.1260/0144598054530011.
Goodenough JB, Park KS. The Li-ion rechargeable battery: a perspective. J Am Chem Soc. 2013;135(4):1167. https://doi.org/10.1021/ja3091438.
Xu Y, Zhou M, Lei Y. Nanoarchitectured array electrodes for rechargeable lithium- and sodium-ion batteries. Adv Mater. 2016;6(10):1502514. https://doi.org/10.1002/aenm.201502514.
Jeong G, Kim YU, Kim H, Kim YJ, Sohn HJ. Prospective materials and applications for Li secondary batteries. Energy Environ Sci. 2011;4(6):1986. https://doi.org/10.1039/C0EE00831A.
Kundu D, Talaie E, Duffort V, Nazar LF. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew Chem Int Ed. 2015;54(11):3431. https://doi.org/10.1002/anie.201410376.
Wu Y, Wu X, Guan Y, Xu Y, Shi F, Liang J. Carbon-based flexible electrodes for electrochemical potassium storage devices. New Carbon Mater. 2022;37(5):852. https://doi.org/10.1016/S1872-5805(22)60631-0.
Wu Y, Chen G, Wu X, Li L, Yue J, Guan Y, Hou J, Shi F, Liang J. Research progress on vanadium oxides for potassium-ion batteries. J Semicond. 2022;43(4):1. https://doi.org/10.1088/1674-4926/44/4/041701.
Wang H, Yang Q, Zheng N, Zhai X, Xu T, Sun Z, Wu L, Zhou M. Roadmap of amorphous metal-organic framework for electrochemical energy conversion and storage. Nano Res. 2022;16(3):4107. https://doi.org/10.1007/s12274-022-5114-8.
Xue H, Gong H, Yamauchi Y, Sasaki T, Ma R. Photo-enhanced rechargeable high-energy-density metal batteries for solar energy conversion and storage. Nano Res Energy. 2022;1(1):9120007. https://doi.org/10.26599/NRE.2022.9120007.
Lv J, Wang B, Hao J, Ding H, Fan L, Tao R, Yang H, Zhou J, Lu B. Single-crystalline Mn-based oxide as a high-rate and long-life cathode material for potassium-ion battery. eScience. 2023;3(1):100081. https://doi.org/10.1016/j.esci.2022.10.007.
Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: a battery of choices. Science. 2011;334(6058):928. https://doi.org/10.1126/science.1212741.
Reddy MV, Subba Rao GV, Chowdari BV. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem Rev. 2013;113(7):5364. https://doi.org/10.1021/cr3001884.
Chen L, Wu H, Ai X, Cao Y, Chen Z. Toward wide-temperature electrolyte for lithium–ion batteries. Battery Energy. 2022;1(2):20210006. https://doi.org/10.1002/bte2.20210006.
Zhou Y, Zhang Z, Zhang H, Li Y, Weng Y. Progress and perspective of vanadium-based cathode materials for lithium ion batteries. Tungsten. 2021;3(3):279. https://doi.org/10.1007/s42864-021-00101-w.
Zeng J, Yang L, Shao R, Zhou L, Utetiwabo W, Wang S, Chen R, Yang W. Mesoscopic Ti2Nb10O29 cages comprised of nanorod units as high-rate lithium-ion battery anode. J Colloid Interface Sci. 2021;600:111. https://doi.org/10.1016/j.jcis.2021.04.136.
Yang L, Zeng J, Zhou L, Shao R, Utetiwabo W, Tufail MK, Wang S, Yang W, Zhang J. Orderly defective superstructure for enhanced pseudocapacitive storage in titanium niobium oxide. Nano Res. 2021;15(2):1570. https://doi.org/10.1007/s12274-021-3703-6.
Zhou X, Wan LJ, Guo YG. Binding SnO2 nanocrystals in nitrogen-doped graphene sheets as anode materials for lithium-ion batteries. Adv Mater. 2013;25(15):2152. https://doi.org/10.1002/adma.201300071.
Nitta N, Wu F, Lee JT, Yushin G. Li-ion battery materials: present and future. Mater Today. 2015;18(5):252. https://doi.org/10.1016/j.mattod.2014.10.040.
Kubota K, Dahbi M, Hosaka T, Kumakura S, Komaba S. Towards K-ion and Na-ion batteries as “beyond Li-ion.” Chem Rec. 2018;18(4):459. https://doi.org/10.1002/tcr.201700057.
Xu J, Xu Y, Lai C, Xia T, Zhang B, Zhou X. Challenges and perspectives of covalent organic frameworks for advanced alkali-metal ion batteries. Sci China Chem. 2021;64(8):1267. https://doi.org/10.1007/s11426-021-1016-6.
Wu Y, Zhang C, Zhao H, Lei Y. Recent advances in ferromagnetic metal sulfides and selenides as anodes for sodium- and potassium-ion batteries. J Mater Chem A. 2021;9(15):9506. https://doi.org/10.1039/D1TA00831E.
Tan H, Feng Y, Rui X, Yu Y, Huang S. Metal chalcogenides: paving the way for high-performance sodium/potassium-ion batteries. Small Methods. 2019;4(1):1900563. https://doi.org/10.1002/smtd.201900563.
Yabuuchi N, Kubota K, Dahbi M, Komaba S. Research development on sodium-ion batteries. Chem Rev. 2014;114(23):11636. https://doi.org/10.1021/cr500192f.
Hosaka T, Kubota K, Hameed AS, Komaba S. Research development on K-ion batteries. Chem Rev. 2020;120(14):6358. https://doi.org/10.1021/acs.chemrev.9b00463.
Li D, Ji F, Liu T, Zhao X, Sun Q, Liu Y, Wang Y, Zhang J, Ci L. Trash to treasure: recycling discarded agarose gel for practical Na/K-ion batteries. J Mater Chem A. 2022;10(28):15026. https://doi.org/10.1039/D2TA02007F.
Feng Y, Lv Y, Fu H, Parekh M, Rao AM, Wang H, Tai X, Yi X, Lin Y, Zhou J, Lu B. Co-activation for enhanced K-ion storage in battery anodes. Natl Sci Rev. 2023;10(7):118. https://doi.org/10.1093/nsr/nwad118.
Shen M, Ding H, Fan L, Rao AM, Zhou J, Lu B. Neuromorphic carbon for fast and durable potassium storage. Adv Funct Mater. 2023;33(25):2213362. https://doi.org/10.1002/adfm.202213362.
Peng J, Yi X, Fan L, Zhou J, Lu B. Molecular extension engineering constructing long-chain organic elastomeric interphase towards stable potassium storage. Energy Lab. 2023;1(2):220014. https://doi.org/10.54227/elab.20220014.
Du Y, Yi Z, Chen B, Xu J, Zhang Z, Bao J, Zhou X. Sn4P3 nanoparticles confined in multilayer graphene sheets as a high-performance anode material for potassium-ion batteries. J Energy Chem. 2022;66:413. https://doi.org/10.1016/j.jechem.2021.08.043.
Xiong J, Yang Z, Guo X, Wang X, Geng C, Sun Z, Xiao A, Zhuang Q, Chen Y, Ju Z. Review on recent advances of inorganic electrode materials for potassium-ion batteries. Tungsten. 2022. https://doi.org/10.1007/s42864-022-00177-y.
Wang X, Wang H. Designing carbon anodes for advanced potassium-ion batteries: materials, modifications, and mechanisms. Adv Powder Mater. 2022;1(4):100057. https://doi.org/10.1016/j.apmate.2022.100057.
Wu X, Leonard DP, Ji X. Emerging non-aqueous potassium-ion batteries: challenges and opportunities. Chem Mater. 2017;29(12):5031. https://doi.org/10.1021/acs.chemmater.7b01764.
Li L, Zheng Y, Zhang S, Yang J, Shao Z, Guo Z. Recent progress on sodium ion batteries: potential high-performance anodes. Energy Environ Sci. 2018;11(9):2310. https://doi.org/10.1039/C8EE01023D.
Lu J, Chen Z, Pan F, Cui Y, Amine K. High-performance anode materials for rechargeable lithium-ion batteries. Electrochem Energy Rev. 2018;1(1):35. https://doi.org/10.1007/s41918-018-0001-4.
Wu Y, Xu R, Wang Z, Hao X, Zhang C, Zhao H, Li W, Wang S, Dong Y, Huang Z, Lei Y. Carbon-free crystal-like Fe1-xS as an anode for potassium-ion batteries. ACS Appl Mater Interfaces. 2021;13(46):55218. https://doi.org/10.1021/acsami.1c17799.
Zhang Y, Zhang L, Lv T, Chu PK, Huo K. Two-dimensional transition metal chalcogenides for alkali metal ions storage. Chemsuschem. 2020;13(6):1114. https://doi.org/10.1002/cssc.201903245.
Li D, Dai L, Ren X, Ji F, Sun Q, Zhang Y, Ci L. Foldable potassium-ion batteries enabled by free-standing and flexible SnS2@C nanofibers. Energy Environ Sci. 2021;14(1):424. https://doi.org/10.1039/D0EE02919J.
Wu Y, Zhang Q, Xu Y, Xu R, Li L, Li Y, Zhang C, Zhao H, Wang S, Kaiser U, Lei Y. Enhanced potassium storage capability of two-dimensional transition-metal chalcogenides enabled by a collective strategy. ACS Appl Mater Interfaces. 2021;13(16):18838. https://doi.org/10.1021/acsami.1c01891.
Du Y, Zhang Z, Xu Y, Bao J, Zhou X. Metal sulfide-based potassium-ion battery anodes: storage mechanisms and synthesis strategies. Acta Phy Chim Sin. 2022;38(11):2205017. https://doi.org/10.3866/PKU.WHXB202205017.
Chhowalla M, Shin HS, Eda G, Li LJ, Loh KP, Zhang H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat Chem. 2013;5(4):263. https://doi.org/10.1038/nchem.1589.
Mohl M, Rautio AR, Asres GA, Wasala M, Patil PD, Talapatra S, Kordas K. 2D tungsten chalcogenides: synthesis, properties and applications. Adv Mater Interfaces. 2020;7(13):2000002. https://doi.org/10.1002/admi.202000002.
Huang J, Wei Z, Liao J, Ni W, Wang C, Ma J. Molybdenum and tungsten chalcogenides for lithium/sodium-ion batteries: beyond MoS2. J Energy Chem. 2019;33:100. https://doi.org/10.1016/j.jechem.2018.09.001.
Wu Y, Yu Y. 2D material as anode for sodium ion batteries: recent progress and perspectives. Energy Storage Mater. 2019;16:323. https://doi.org/10.1016/j.ensm.2018.05.026.
Xu Y, Bahmani F, Zhou M, Li Y, Zhang C, Liang F, Kazemi SH, Kaiser U, Meng G, Lei Y. Enhancing potassium-ion battery performance by defect and interlayer engineering. Nanoscale Horiz. 2019;4(1):202. https://doi.org/10.1039/C8NH00305J.
Dong Y, Xu Y, Li W, Fu Q, Wu M, Manske E, Kroger J, Lei Y. Insights into the crystallinity of layer-structured transition metal dichalcogenides on potassium ion battery performance: a case study of molybdenum disulfide. Small. 2019;15(15):1900497. https://doi.org/10.1002/smll.201900497.
Dong H, Xu Y, Zhang C, Wu Y, Zhou M, Liu L, Dong Y, Fu Q, Wu M, Lei Y. MoS2 nanosheets with expanded interlayer spacing for enhanced sodium storage. Inorg Chem Front. 2018;5(12):3099. https://doi.org/10.1039/C8QI00969D.
Eftekhari A. Tungsten dichalcogenides (WS2, WSe2, and WTe2): materials chemistry and applications. J Mater Chem A. 2017;5(35):18299. https://doi.org/10.1039/C7TA04268J.
Fan S, Zou X, Du H, Gan L, Xu C, Lv W, He Y-B, Yang Q-H, Kang F, Li J. Theoretical investigation of the intercalation chemistry of lithium/sodium ions in transition metal dichalcogenides. J Phys Chem C. 2017;121(25):13599. https://doi.org/10.1021/acs.jpcc.7b05303.
Zhang R, Bao J, Pan Y, Sun CF. Highly reversible potassium-ion intercalation in tungsten disulfide. Chem Sci. 2019;10(9):2604. https://doi.org/10.1039/C8SC04350G.
Wu Y, Xu Y, Li Y, Lyu P, Wen J, Zhang C, Zhou M, Fang Y, Zhao H, Kaiser U, Lei Y. Unexpected intercalation-dominated potassium storage in WS2 as a potassium-ion battery anode. Nano Res. 2019;12(12):2997. https://doi.org/10.1007/s12274-019-2543-0.
Geng S, Zhou T, Jia M, Shen X, Gao P, Tian S, Zhou P, Liu B, Zhou J, Zhuo S, Li F. Carbon-coated WS2 nanosheets supported on carbon nanofibers for high-rate potassium-ion capacitors. Energy Environmental Sci. 2021;14(5):3184. https://doi.org/10.1039/D1EE00193K.
Mu Z, Gao S, Huo S, Zhao K. Mixed-phase 1T/2H-WS2 nanosheets on N-doped multichannel carbon nanofiber as current collector-integrated electrode for potassium battery anode. J Colloid Interface Sci. 2023;630:823. https://doi.org/10.1016/j.jcis.2022.10.065.
Lei Z, Zheng J, He X, Wang Y, Yang X, Xiao F, Xue H, Xiong P, Wei M, Chen Q, Qian Q, Zeng L. Defect-rich WS2–SPAN nanofibers for sodium/potassium-ion batteries: ultralong lifespans and wide-temperature workability. Inorg Chem Front. 2023;10(4):1187. https://doi.org/10.1039/D2QI02483G.
Zhu Q, Li W, Wu J, Tian N, Li Y, Yang J, Liu B, Jiang J. Vacancy engineering in WS2 nanosheets for enhanced potassium-ion storage. J Power Sources. 2022;542:231791. https://doi.org/10.1016/j.jpowsour.2022.231791.
Zhu Q, Li W, Wu J, Tian N, Li Y, Yang J, Liu B. Filling selenium into sulfur vacancies in ultrathin tungsten sulfide nanosheets for superior potassium storage. ACS Appl Mater Interfaces. 2022;14(46):51994. https://doi.org/10.1021/acsami.2c16173.
Li Z, Yuan F, Han M, Yu J. Fast K-ion storage enabled by N, O Co-doping and atomic-interface engineering on WS2. Chem Eng J. 2022;450(4):138451. https://doi.org/10.1016/j.cej.2022.138451.
Zhang Z, Yang M, Zhao N, Wang L, Li Y. Two-dimensional transition metal dichalcogenides as promising anodes for potassium ion batteries from first-principles prediction. Phys Chem Chem Phys. 2019;21(42):23441. https://doi.org/10.1039/C9CP03948A.
Jiao X, Liu X, Wang B, Wang G, Wang X, Wang H. A controllable strategy for the self-assembly of WM nanocrystals/nitrogen-doped porous carbon superstructures (M = O, C, P, S, and Se) for sodium and potassium storage. J Mater Chem A. 2020;8(4):2047. https://doi.org/10.1039/C9TA11312F.
Kang B, Chen X, Zeng L, Luo F, Li X, Xu L, Yang MQ, Chen Q, Wei M, Qian Q. In situ fabrication of ultrathin few-layered WSe2 anchored on N, P dual-doped carbon by bioreactor for half/full sodium/potassium-ion batteries with ultralong cycling lifespan. J Colloid Interface Sci. 2020;574:217. https://doi.org/10.1016/j.jcis.2020.04.055.
Xing L, Han K, Liu Q, Liu Z, Chu J, Zhang L, Ma X, Bao Y, Li P, Wang W. Hierarchical two-atom-layered WSe2/C ultrathin crumpled nanosheets assemblies: engineering the interlayer spacing boosts potassium-ion storage. Energy Storage Mater. 2021;36:309. https://doi.org/10.1016/j.ensm.2021.01.005.
Chen X, Muheiyati H, Sun X, Zhou P, Wang P, Ding X, Qian Y, Xu L. Rational design of tungsten selenide@N-doped carbon nanotube for high-stable potassium-ion batteries. Small. 2022;18(5):2104363. https://doi.org/10.1002/smll.202104363.
Zhao Z, Xu T, Yu X. Unlock the potassium storage behavior of single-phased tungsten selenide nanorods via large cation insertion. Adv Mater. 2023;35(5):2208096. https://doi.org/10.1002/adma.202208096.
Soares DM, Singh G. Superior electrochemical performance of layered WTe2 as potassium-ion battery electrode. Nanotechnology. 2020;31(45):455406. https://doi.org/10.1088/1361-6528/ababcc.
Soares DM, Singh G. Weyl semimetal orthorhombic Td-WTe2 as an electrode material for sodium- and potassium-ion batteries. Nanotechnology. 2021;32(50):505402. https://doi.org/10.1088/1361-6528/ac23f3.
Li D, Sun Q, Zhang Y, Chen L, Wang Z, Liang Z, Si P, Ci L. Surface-confined SnS2@C@rGO as high-performance anode materials for sodium- and potassium-ion batteries. ChemSusChem. 2019;12(12):2689. https://doi.org/10.1002/cssc.201900719.
Sun Q, Li D, Dai L, Liang Z, Ci L. Structural engineering of SnS2 encapsulated in carbon nanoboxes for high-performance sodium/potassium-ion batteries anodes. Small. 2020;16(45):2005023. https://doi.org/10.1002/smll.202005023.
Zhou J, Liu Y, Zhang S, Zhou T, Guo Z. Metal chalcogenides for potassium storage. InfoMat. 2020;2(3):437. https://doi.org/10.1002/inf2.12101.
Yao Q, Zhu C. Advanced post-potassium-ion batteries as emerging potassium-based alternatives for energy storage. Adv Funct Mater. 2020;30(49):2005209. https://doi.org/10.1002/adfm.202005209.
Wang F, Han F, He Y, Zhang J, Wu H, Tao J, Zhang C, Zhang F, Liu J. Unraveling the voltage failure mechanism in metal sulfide anodes for sodium storage and improving their long cycle life by sulfur-doped carbon protection. Adv Funct Mater. 2020;31(3):2007266. https://doi.org/10.1002/adfm.202007266.
Cao L, Luo B, Xu B, Zhang J, Wang C, Xiao Z, Li S, Li Y, Zhang B, Zou G, Hou H, Ou X, Ji X. Stabilizing intermediate phases via efficient entrapment effects of layered VS4/SnS@C heterostructure for ultralong lifespan potassium-ion batteries. Adv Funct Mater. 2021;31(36):2103802. https://doi.org/10.1002/adfm.202103802.
Yang G, Wu Y, Fu Q, Zhao H, Lei Y. Nanostructured metal selenides as anodes for potassium-ion batteries. Sustain Energy Fuels. 2022;6(9):2087. https://doi.org/10.1039/D2SE00067A.
Wei X, Wang X, Tan X, An Q, Mai L. Nanostructured conversion-type negative electrode materials for low-cost and high-performance sodium-ion batteries. Adv Funct Mater. 2018;28(46):1804458. https://doi.org/10.1002/adfm.201804458.
Liu H, Su D, Wang G, Qiao SZ. An ordered mesoporous WS2 anode material with superior electrochemical performance for lithium ion batteries. J Mater Chem. 2012;22(34):17437. https://doi.org/10.1039/C2JM33992G.
Liu Y, Zhang N, Kang H, Shang M, Jiao L, Chen J. WS2 nanowires as a high-performance anode for sodium-ion batteries. Chem Eur J. 2015;21(33):11878. https://doi.org/10.1002/chem.201501759.
Srinivaas M, Wu CY, Duh JG, Wu JM. Highly rich 1T metallic phase of few-layered WS2 nanoflowers for enhanced storage of lithium-ion batteries. ACS Sustain Chem Eng. 2019;7(12):10363. https://doi.org/10.1021/acssuschemeng.9b00351.
Chen R, Li S, Liu J, Li Y, Ma F, Liang J, Chen X, Miao Z, Han J, Wang T, Li Q. Hierarchical Cu doped SnSe nanoclusters as high-performance anode for sodium-ion batteries. Electrochim Acta. 2018;282:973. https://doi.org/10.1016/j.electacta.2018.07.035.
Han G, Chen ZG, Ye D, Yang L, Wang L, Drennan J, Zou J. In-doped Bi2Se3 hierarchical nanostructures as anode materials for Li-ion batteries. J Mater Chem A. 2014;2(19):7109. https://doi.org/10.1039/C4TA00045E.
Zou Z, Wang X, Huang J, Wu Z, Gao F. An Fe-doped nickel selenide nanorod/nanosheet hierarchical array for efficient overall water splitting. J Mater Chem A. 2019;7(5):2233. https://doi.org/10.1039/C8TA11072G.
He H, Huang D, Gan Q, Hao J, Liu S, Wu Z, Pang WK, Johannessen B, Tang Y, Luo JL, Wang H, Guo Z. Anion vacancies regulating endows MoSSe with fast and stable potassium ion storage. ACS Nano. 2019;13(10):11843. https://doi.org/10.1021/acsnano.9b05865.
Xiong P, Wu Y, Liu Y, Ma R, Sasaki T, Wang X, Zhu J. Two-dimensional organic–inorganic superlattice-like heterostructures for energy storage applications. Energy Environ Sci. 2020;13(12):4834. https://doi.org/10.1039/D0EE03206A.
Wang W, Bao J, Sun C. Liquid-phase exfoliated WS2-graphene composite anodes for potassium-ion batteries. Chinese J Struct Chem. 2020;39:493. https://doi.org/10.14102/j.cnki.0254-5861.2011-2457.
Ghosh S, Qi Z, Wang H, Martha SK, Pol VG. WS2 anode in Na and K-ion battery: effect of upper cut-off potential on electrochemical performance. Electrochim Acta. 2021;383:138339. https://doi.org/10.1016/j.electacta.2021.138339.
Chen M, Zhao J, Sun C. High-volumetric-capacity WSe2 anode for potassium-ion batteries. Chinese J Struct Chem. 2020;40(7):926. https://doi.org/10.14102/j.cnki.0245-5861.2011-3106.
Liu YR, Lei ZW, Liu RP, Li XY, Xiong PX, Luo YJ, Chen QH, Wei MD, Zeng LX, Qian QR. Sn-doped induced stable 1T-WSe2 nanosheets entrenched on N-doped carbon with extraordinary half/full sodium/potassium storage performance. Rare Met. 2023;42(5):1557. https://doi.org/10.1007/s12598-022-02174-z.
Yu L, He X, Peng B, Wang W, Wan G, Ma X, Zeng S, Zhang G. Constructing ion diffusion highway in strongly coupled WSe2-carbon hybrids enables superior energy storage performance. Matter. 2023;6(5):1604. https://doi.org/10.1016/j.matt.2023.03.013.
Zhang C, Wang F, Han F, Wu H, Zhang F, Zhang G, Liu J. Improved electrochemical performance of sodium/potassium-ion batteries in ether-based electrolyte: cases study of MoS2@C and Fe7S8@C anodes. Adv Mater Interfaces. 2020;7(13):2000486. https://doi.org/10.1002/admi.202000486.
Hu M, Ju Z, Bai Z, Yu K, Fang Z, Lv R, Yu G. Revealing the critical factor in metal sulfide anode performance in sodium-ion batteries: an investigation of polysulfide shuttling issues. Small Methods. 2019;4(1):1900673. https://doi.org/10.1002/smtd.201900673.
Xiao N, McCulloch WD, Wu Y. Reversible dendrite-free potassium plating and stripping electrochemistry for potassium secondary batteries. J Am Chem Soc. 2017;139(28):9475. https://doi.org/10.1021/jacs.7b04945.
Bhide A, Hofmann J, Durr AK, Janek J, Adelhelm P. Electrochemical stability of non-aqueous electrolytes for sodium-ion batteries and their compatibility with Na0.7CoO2. Phys Chem Chem Phys. 2014;16(5):1987. https://doi.org/10.1039/C3CP53077A.
Hu Z, Zhu Z, Cheng F, Zhang K, Wang J, Chen C, Chen J. Pyrite FeS2 for high-rate and long-life rechargeable sodium batteries. Energy Environ Sci. 2015;8(4):1309. https://doi.org/10.1039/C4EE03759F.
Li S, Zhang W, Zheng J, Lv M, Song H, Du L. Inhibition of polysulfide shuttles in Li–S batteries: modified separators and solid-state electrolytes. Adv Energy Mater. 2020;11(2):2000779. https://doi.org/10.1002/aenm.202000779.
Yang X, Li X, Adair K, Zhang H, Sun X. Structural design of lithium-sulfur batteries: from fundamental research to practical application. Electrochem Energy Rev. 2018;1(3):239. https://doi.org/10.1007/s41918-018-0010-3.
Zhang S, Ueno K, Dokko K, Watanabe M. Recent advances in electrolytes for lithium-sulfur batteries. Adv Energy Mater. 2015;5(16):1500117. https://doi.org/10.1002/aenm.201500117.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 52002094), the Shenzhen Steady Support Plan (GXWD20221030205923001), the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515110756), the Shenzhen Science and Technology Program (Grant Nos. JCYJ20210324121411031, JSGG202108021253804014, RCBS20210706092218040), the Open Fund of the Guangdong Provincial Key Laboratory of Advanced Energy Storage Materials (Grant. No. asem202107), and the Shenyang University of Technology (QNPY202209-4).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, YH., Xia, WH., Liu, YZ. et al. Tungsten chalcogenides as anodes for potassium-ion batteries. Tungsten 6, 278–292 (2024). https://doi.org/10.1007/s42864-023-00237-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42864-023-00237-x