Abstract
Background
Polycystic ovary syndrome (PCOS) is the most common feminine endocrine disorder, characterized by androgen excess, ovulatory dysfunction, and polycystic ovarian morphology. The negative impact of symptoms on the quality of life (QoL) of patients is still not clear.
Purpose
The present review aimed at studying the impact of the symptoms, the psychological symptoms, and brain alterations in women with PCOS.
Methods
A systematic search was undertaken for studies that assessed the impact of PCOS symptoms on QoL, psychological symptoms, and brain alterations in PCOS patients.
Results
Most of the information about QoL came from psychometric studies, which used culture-based questionnaires. Alterations of sleep quality, body image, and mood disorders can negatively affect the QoL of the patients. Sexual satisfaction and desire were affected by PCOS. Brain imaging studies showed functional alterations that are associated with impairments of visuospatial working memory, episodic and verbal memory, attention, and executive function.
Conclusions
Several factors can negatively influence the quality of life of the patients, and they are directly related to hyperandrogenism and the risk of infertility. In particular, obesity, hirsutism, acne, and the fear of infertility can have a direct impact on self-esteem and sexual function. Metabolic and psychiatric comorbidities, such as mood, anxiety, and eating disorders, can affect the well-being of the patients. Moreover, specific cognitive alterations, such as impairments in attention and memory, can limit PCOS patients in a series of aspects of daily life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is a multifaceted endocrine, reproductive, and metabolic condition [1, 2]. At present, PCOS is considered to be the most common feminine endocrine disorder during reproductive age [3] and is characterized by androgen excess, ovulatory dysfunction, and polycystic ovarian morphology (PCOM) [4]. The most recent international guidelines require the presence of two or more of the three previously mentioned criteria to establish a diagnosis of PCOS, after the exclusion of other related endocrine disorders [5]. The most common forms of presentation include irregular menstrual cycles and hirsutism [6], but acanthosis nigricans [7] and increased risk of infertility [8] should also be considered. According to the population of reference sample size and body mass index, the worldwide impact of PCOS varies from 5–15% [9] up to 20% of female patients of reproductive age [1].
PCOS is a complex syndrome associated with psychiatric comorbidities, neurocognitive and sexual alteration as shown by several studies performed during the last years. This condition can negatively affect the quality of life of the patients. Despite the wider use of the term quality of life (QoL) or health-related quality of life (HRQoL) in clinical research and medicine, there is no clear methodological and conceptual definition [10]. Recently, QoL was defined as a multidimensional outcome involving the physical, emotional, and socioeconomic well-being of one's life [11].
During the last few years, several studies have attempted to disentangle the negative impact of PCOS on several cognitive and emotional functions relevant to the quality of life of patients. Studies that used specific psychometric instruments assessed the impact and the presence of specific symptoms, providing information that can be useful, not only to assess the physical and emotional well-being in a specific culture but also to stimulate the implementation of new therapies and trials. Most of these instruments were adapted to different cultures.
Similarly, PCOS is comorbid with psychiatric disorders, such as anxiety and depression. The impact of these disorders on the quality of life of psychiatric patients, without other comorbidities, has been widely studied. Thus, studies providing information about neuropsychiatric symptoms in PCOS can allow us to characterize the dimensions of life that are more affected by the syndrome.
The present review aims to characterize the state of the art of the principal psychometric instruments that are useful to provide clinical information about the dimensions of life that can be affected in PCOS patients. Also, following what was mentioned above, studies about psychological symptoms, alterations in sexual behavior, and functional and structural brain alterations, as assessed by neuroimaging techniques, will be discussed.
Materials and methods
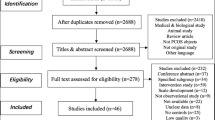
Despite the narrative nature of the present review, our research question was based on the PICO strategy. Specifically, we were interested in analyzing the published literature that included studies involving PCOS women (P; Population), to study the symptoms and alterations as mentioned above (O; Outcome) with the use of questionnaires and imaging or psychophysiological techniques (I; Intervention) in comparison with healthy women (C; Comparison). To this purpose, we used terms related to the PICO strategy to perform an electronic search in the principal databases, such as MEDLINE, Scopus, and Web of Science.
To the same objective, previously published meta-analyses and narrative or systematic reviews were also retrieved to check the reported bibliographic references. In this way, the studies of interest have been retrieved and the content has been assessed.
Diagnostic criteria
Due to the heterogeneous and multifactorial nature of PCOS symptoms, a lack of a clear, agreed-upon definition and diagnostic criteria is still present [12]. The diagnostic criteria have been recurrently changing, thus generating uncertainty on the type of PCOS that was considered in each study. The National Institutes of Health (NIH) proposed diagnostic criteria for PCOS, which included an association of chronic anovulation with androgen excess [13]. In 2004, in a meeting of the European Society of Human Reproduction and Embryology (ESHRE) with the American Society for Reproductive Medicine (ASRM), a new definition of PCOS was proposed. This definition is nowadays the most widely accepted. According to this consensus, two of the following criteria, the presence of hyperandrogenism, chronic ovulatory dysfunction, and ultrasound characteristics of polycystic ovaries, were relevant for diagnosis [14].
In 2006, the Androgen Excess Society (AES) proposed a different definition of PCOS as a disorder mostly characterized by androgen excess, e.g., the presence of clinical and/or biochemical hyperandrogenism together with ovulatory dysfunction, identified by either oligoanovulation or PCOM [8].
To maximize the comparability in research, the NIH Evidence-based Methodology Workshop Panel on PCOS recommended maintaining the comprehensive and inclusive diagnostic criteria of ESHRE/ASRM 2003 and classifying PCOS into four phenotypes [15]. Phenotypes A and B are often called “classic PCOS”, that is women with hyperandrogenism, ovulatory dysfunction, and with, in the case of phenotype A, or without, for phenotype B, PCOM. Phenotype C, or “ovulatory PCOS”, is distinguished by hyperandrogenism and PCOM without ovulatory dysfunction. In phenotype D, “non-hyperandrogenic PCOS”, women have ovulatory dysfunction and PCOM without hyperandrogenism.
Psychological impact of PCOS
Since 2014, great attention has been paid to the comorbidities shown in PCOS [16]. PCOS women show increased odds of depressive and anxiety symptoms [16,17,18,19,20] (Table 1), in addition to a decreased QoL [18, 19, 21, 22] (Table 2). According to a recent meta-analysis, most of the published studies related to PCOS were focused on anxiety and depression [23]. Chaudhari et al. found that 38.6% of anxiety in their sample of PCOS is associated with infertility and alopecia, as well as 25.7% of depression being related to acne [24]. The assessment of depressive and anxious symptoms is very relevant. Nevertheless, the use of anxiety and depression-dedicated measures, such as Beck anxiety (BAI) [25] and depression inventories (BDI) [26], Hospital Anxiety and Depression Scale (HADS) [27], Hamilton Anxiety Rating Scale (Ham-A) [28] and Hamilton Depression Rating Scale (Ham-D) [29], seems to perform even better and may be preferable in the assessment of depression and anxiety in these patients.
However, Brucotao et al., highlighted the relevance of other psychiatric comorbidities, showing increased rates of obsessive–compulsive disorder and somatization in women with PCOS [23]. Apart from somatization disorders, a few studies reported the presence of bipolar and obsessive–compulsive disorders [30]. Interestingly, somatization disorders in PCOS can be related to physical symptoms and alteration of body appearance, such as in patients with hirsutism, which can create psychological distress. Some women with PCOS even reported feeling ‘different’ from other women and ‘less’ feminine’ [31]. PCOS also showed a higher lifetime incidence of social phobia and eating disorders [18, 21, 32] like bulimia nervosa and binge eating [33, 34]. Furthermore, PCOS reports higher rates of body dysmorphic disorder. Additionally, patients report poor body image, weight stigma, seven times more common chance of suicide, along with a higher current and lifetime use of antidepressants and anxiolytic drugs [21].
Moreover, a higher prevalence of sleeping disorders has been reported in PCOS [35], with disorders like hypersomnia and obstructive sleep apnea [34, 36, 37]. PCOS was associated with lower sleep quality in the presence of greater luteinizing hormone (LH) and serum insulin levels [38]. Indeed, higher LH levels were found to be significantly correlated with indices of poor sleep quality [39] and LH pulses occurred most frequently in association with brief awakenings, during periods of sleep [40]. Furthermore, in women with high BMI levels, sleep quality was also associated with insulin resistance, with a significantly lower total score of sleep quality in the insulin-resistant group than in the non-insulin-resistant group [41]. In both women with and without PCOS, insulin resistance was considered the strongest risk factor for sleep apnea, after controlling for age, BMI, and testosterone levels [42]. Similarly, insulin resistance markers are related to short sleep duration and even prior diagnosis of obstructive sleep apnea [43].
Psychiatric disorders represent one of the most common comorbidities in PCOS. Among these, anxiety and depression represent the most frequent, followed by eating and somatization disorders. However, recent studies also found an association between PCOS and sleep disorders.
Clinical assessment and quality of life
The assessment of symptoms that can negatively affect the QoL is considered extremely relevant for the diagnosis and treatment of PCOS. Moreover, a few studies found that both NIH and non-NIH phenotypes of PCOS have exhibited similar psychological profiles. Milder phenotypes of the syndrome can still be associated with alteration in several cognitive domains [44]. Hence, psychological function and QoL should be taken into consideration in all PCOS patients [16].
Health-related quality of life (HRQoL) has been studied by some generic questionnaires, with the Short Form-36 questionnaire (SF-36) [45] being the most acknowledged and most often used instrument to measure HRQoL in various medical, including PCOS [46]. The results using S-36 have been fairly consistent in suggesting a reduction in HRQoL in PCOS patients. According to Bazarganipour et al., PCOS affected all domains in the SF-36, and psychological domains, such as the emotional and vitality ones, were the most negatively impacted by PCOS [47].
Other previous studies showed that the most affected domains in SF-36 were the ones related to emotional problems [48, 49].
Several specific psychometric questionnaires have been developed and validated to assess the QoL in PCOS women.
Cronin et al. developed a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome [50]. PCOSQ includes a total of 26 items and five subscales including emotions, body hair, weight, infertility, and menstrual problems. Later, the questionnaire was modified (M-PCOSQ) to include an acne subscale [50, 51].
In 2016, PCOSQ-50, developed by Nasiri-Amiri et al., is a new and comprehensive way of measuring QoL in PCOS women, including the sexual domain, which had been overlooked by previous questionnaires [52]. The PCOSQ-50 incorporated 50 items in a 5-point Likert scale representing six areas, namely emotion, obesity and menstrual disorders, fertility, sexual function, hirsutism, and coping.
According to Williams et al., a more sensitive PCOS quality-of-life measure was needed for clinical and research settings [53]. Therefore, they developed the 35-item polycystic ovary syndrome quality of life scale (PCOSQOL) that comprises the impact of PCOS, infertility, hirsutism, and mood subscales.
Nonetheless, even with the previous questionnaires, there was still an absence of a reliable and validated HRQoL questionnaire to measure the impact of PCOS on several life aspects of other cultures, religions, or ethnicities. This was the case for instance of Arabic women where, in particular, one would need to address sexuality in married women only. As such, Odhaib et al. developed two distinct questionnaires in Arabic for married and unmarried women with PCOS for effective QoL evaluation, PCOSQoL-47 and PCOSQoL-42 [54]. For married women with PCOS, PCOSQoL-47 comprehends five subscales, such as psychological and emotional status, fertility and sexual life, body image, hair disorders, and acne domain, as well as obesity and menstrual disorders. On the other hand, for unmarried women, PCOSQoL-42 also consists of five subscales, including the psychological and emotional domain, menstrual disorders and fertility domain, body image domain, hair disorders and acne domain, and coping domain. The M-PCOSQ was also adapted and validated for Iranian PCOS patients, which showed a prevalence of 11% in the total population [55]. Despite the Iranian validation confirming that the conceptualization of QoL is universal, the questionnaire was modified concerning the original version, with an item that was found loading in the menstruation disturbances scale instead of in the emotion subscale. These questionnaires may facilitate further studies in Arabic and Persian-speaking communities and other communities with similar social norms concerning marriage and sexuality.
Of all the HRQoL-specific questionnaires for PCOS, PCOSQ has been the most frequently used in most studies and clinical practice [44]. Both the PCOSQ and its modified variant showed adequate content and construct validity, reliability, and internal consistency [56]. Similar psychometric properties were shown in the German-adapted version, the PCOSQ-G [57]. In terms of structural validity, a few studies suggest that they have an additional dimension associated with menstruation, in addition to its existing dimensions [51, 58]. However, in a sample of Serbian women affected by PCOS, who participated in a validation study for PCOSQ-50 [59], the most relevant affected dimensions were hirsutism, obesity, and menstrual disorders. As stated by the authors, PCOSQ-50 is a useful and valid instrument to assess the HRQoL in women with PCOS, showing good psychometric properties.
A meta-analysis, which assessed the homogeneity of the scores of each domain of PCOSQ/MPCOSQ, found that the most significantly affected domains of HRQoL in PCOS were hirsutism and menstruation [60]. Whereas in the general population, hirsutism affects 4–11% of women, in PCOS, its prevalence is estimated to be 65–75% [9, 61].
Cultural factors are important variables that can be taken into consideration for the assessment of QoL. However, HRQoL may vary across populations according to the discrepancy in psychosocial factors such as cultural heritage, family structure, medical systems, and norms related to illness-related communication. Predictably, cultural variables seem to influence responses to different dimensions of symptoms. For instance, compared to Austrian women, Brazilian women demonstrated considerably more distress related to hirsutism, menstrual irregularities, and infertility [62], while Islamic immigrant women expressed significantly more concern about menstrual irregularities and infertility [63].
These results were confirmed by a cross-sectional study of 300 women with PCOS that found menstruation and infertility to be the HRQoL aspects more affected by PCOS [64]. Thus, in cultures in which there is a higher expectation of women having children, it would be expected that infertility would have a notably negative impact on QoL.
The assessment of quality of life and health-related quality of life in PCOS showed that specific and patient-based psychometric instruments developed in the last years are valid and reliable instruments. They can be useful in both clinical and research settings. Nonetheless, cultural differences need to be taken into account during the clinical assessment performed with one or more of the above-described scales.
Sexuality and sexual behavior
The research on the psychosocial aspects of PCOS has only recently emerged, and data on sexual function in women with PCOS is exiguous and often contradicting. Female sexual function (FSF) is an intricate biopsychosocial phenomenon that can be influenced by many factors. It can be impaired by metabolic syndrome [65], obesity [66, 67], infertility [68], mental health [69,70,71,72], self-esteem [73, 74], body image [75], and body awareness [76].
These factors also occur frequently in PCOS and may also promote sexual dysfunction. Sexual dysfunction refers to difficulties that arise during the sexual response cycle which prevent the individual from experiencing satisfaction from sexual activity. Female sexual dysfunction (FSD) is a significant public health problem that affects 41% of premenopausal women [77]. Not only can high rates of sexual dysfunction be found in PCOS [78], but PCOS younger than 30 years are 40% more likely to have FSD than women without PCOS [79]. This frequent coexistence should not be neglected in clinical practice. In addition, a tendency was found for PCOS to report more dyspareunia and lack of sexual satisfaction [79].
As it is well known, androgens seem to play a considerable role in sexual desire (SD). Despite the role of modulation of sexual desire played by ovarian steroids, disagreement remains about the strength of the effect attributable to ovarian estradiol or testosterone in increasing SD in women [80].
Low levels of androgen showed a negative impact on SD [81,82,83,84]. However, it is still not clear if ovarian or adrenal androgens can play a different role in SD. In fact, besides having increased LH, as well as LH/FSH ratio, PCOS is characterized by high levels of dehydroepiandrosterone and androstenedione of adrenal origin as well as androstenedione and testosterone of ovarian origin [85].
PCOS and Nonclassic Congenital Adrenal Hyperplasia (NC-CAH) patients are both characterized by markedly increased production of androgens [86]. However, in NC-CAH, low sexual desire has been correlated with hirsutism and testosterone levels hence demonstrating that other factors besides androgens must also be active players in the development of sexual desire.
Although it is not easy to define blood androgen levels that can be considered as androgen deficiency, short-term clinical trials suggest that low-dose testosterone therapy in women with hypoactive sexual desire disorder can improve sexual function and satisfaction [82, 83]. Reciprocally, women suffering from hyperandrogenism can also benefit from the use of anti-androgenic oral contraception to improve sexual and social self-esteem [87].
Wåhlin-Jacobsen et al. found that in women between 25 and 44 years without systematic usage of hormonal contraception, SD was correlated with total testosterone, free testosterone, androstenedione, and dehydroepiandrosterone sulfate (DHEAS) [88]. In women aged between 45 and 65 years, SD was only associated with androstenedione. Nonetheless, clinical signs suggest that sensitivity to androgen levels, more than actual androgen levels, may be associated with some aspects of sexual desire [89].
According to Pastoor et al., PCOS women report substantially lower scores in sexual function, especially in arousal, lubrication, and orgasm scores [90]. PCOS women also indicated a greater decrease in terms of sexual attractiveness, satisfaction with their sex life, and a significant impact of their physical appearance and body hair on sexuality. Even though the arousal and lubrication subscales reported considerably inferior scores in the Female Sexual Function Index (FSFI), Zhao et al. found no substantial association between FSD and PCOS [91].
Therefore, it remains difficult to determine the association between androgens and sexual function in PCOS. Furthermore, menstrual irregularities and subfertility could also lead to loss of self-esteem and emotional distress, such as depression and anxiety, which could adversely affect the satisfaction of sexual relationships [92, 93]. Due to obesity and excess androgens, PCOS can find themselves less appealing because of dissatisfaction with their body and the potential loss of feminine identity [94]. Moreover, treatment with metformin for 6 months has reduced dyspareunia and improved sexual satisfaction and frequency attributable to increased insulin sensitivity [95]. This could be related to the known strong association of diabetes mellitus with FSD [96]. However, it can also be related to the positive impact that metformin use has in PCOS in terms of reducing body weight and BMI, total testosterone, androstenedione, fasting blood glucose, and increasing the likelihood of pregnancy [97], which leads to an increased QoL. Another hormone that plays a complex role in human sexuality is oxytocin [98].
Similarly, an interesting study that was done in a PCOS animal model demonstrated that endogenous/exogenous oxytocin showed positive results in the reduction of body weight. Indeed, this neuropeptide, synthesized in the hypothalamus, is involved in controlling metabolism, appetite, and body weight and the PCOS rats showed obesity and low levels of endogenous Oxytocin. After OT administration, PCOS rats showed a decrease in body weight [99]. These results can be promising, stimulate clinical trials in PCOS patients, and maybe result in an improvement of body image and sexual functions. Nevertheless, these results should be treated with caution since we have no information about the embodied image of rats. Despite this pioneering study, the polymorphisms of the gene of the Oxytocin receptor (OXTR) are associated with the development of insulin resistance and body weight [100, 101]. However, a recent population study reported five novel OXTR variants significantly associated with the risk of developing PCOS in multigenerational Italian families [102].
Women with excess androgens treated with anti-androgenic contraceptives experienced a significant improvement in hirsutism, sexual pain, orgasm, and satisfaction [87, 103]. Yet, other studies found no changes related to sexual desire levels even after normalizing androgen levels in PCOS [87].
Perturbations of sexual functions in PCOS involve the sexual satisfaction and desire dimensions. However, physical alterations, such as hirsutism or overweight and metabolic disorders can play a crucial role in sexual dysfunctions. Although these symptoms can be caused by hyperandrogenism, the role of androgens and anti-androgenic therapies in female sexual functions should be carefully assessed in future studies.
Brain alterations in PCOS
Thanks to the recent advances in brain imaging techniques, such as structural (MRI) and functional magnetic resonance imaging (fMRI) or positron emission tomography (PET), it was possible to investigate the effect of hyperandrogenism on the female brain and related cognitive and emotional functions [104]. Several studies investigated gender differences in terms of cognition and emotion and simultaneously assessed brain responses. Similarly, during the last few years, electroencephalography (EEG) has provided complementary information about brain regions and the functions affected by hormonal alteration in PCOS. Although only a few studies investigated the brain alterations related to PCOS using neuroimaging techniques and EEG, several evidence found that PCOS is associated with poorer cognitive functioning (Table 3).
According to Lai et al., PCOS induces functional alteration in brain regions responsible for visual working memory, such as the right middle and superior frontal gyrus [105]. The alteration found in these two frontal regions is also associated with a specific deficit of the attentional component of working memory. Similarly, the functional connectivity between the middle and superior frontal gyrus showed a negative correlation with LH levels and with the LH/FSH ratios. Notably, previous studies found that PCOS women had worse performance on visuospatial working memory [106]. In addition, more general visuospatial ability [107] and visuospatial learning [108] are negatively affected by PCOS. An fMRI study analyzed the effects of overexposure to androgens and subsequent antiandrogenic treatment on brain activity during working memory processing [109]. They reported a higher activation within the right superior and inferior parietal lobe in POCS during the task. After the treatment, no between-group difference in the global brain activity was found and PCOS showed better accuracy in high memory load conditions during working memory tasks. Thus, PCOS may need more neural resources during working memory tasks, having less efficient executive function [109].
These alterations in working memory confirmed previous findings showing episodic memory [110] and executive function [107] impairments in PCOS patients.
Li et al. also found higher executive functions and memory impairments [38]. Besides, since PCOS was associated with greater LH levels [38] (Table 4), they likewise found correlations between serum hormone alterations and cognitive function scores.
A lower functional connectivity value, as measured using the amplitude of low-frequency fluctuation (ALFF), in the left middle frontal gyrus [38]. The left middle frontal gyrus is related to poor executive function and depressive disorders [38] and it showed a negative correlation with plasma insulin levels in insulin-resistant PCOS. A higher functional connectivity strength between the left middle and inferior frontal gyrus, which are correspondingly associated with the development of literacy [111] and language processing [112], was positively correlated with serum testosterone (free T) levels. It was also reported a negative correlation of LH plasma levels with a higher functional connectivity strength between the left posterior cingulate gyrus, a central node in the Default Mode Network [113, 114], and the pars triangularis of the left inferior frontal gyrus, which is related to language processing [115]. Finally, a higher functional connectivity strength was found with the right middle occipital gyrus associated with visuospatial processing [116], and the right inferior occipital gyrus, which is associated with impaired memory [38] (Fig. 1).
Resulting brain clusters from previously published fMRI studies on PCOS. The figure depicts a tridimensional map of the clusters as reported in previously published fMRI studies [38, 105, 109, 119,120,121,122,123]. The map was overimposed on an MNI (Montreal Neurological Institute) template in neurological convention. The map was created using Ginger Ale software (http://brainmap.org), and the 3D glass brain template was built using Mango software
PCOS women have shown significantly lower performance on the tests of attention [107] and psychomotor speed than healthy women [108].
PCOS patients showed decreased neuropsychological performance scores [20] as measured by the Montreal Cognitive Assessment (MoCA) [117]. Then, participants showed less absolute alpha power, more frontal beta waves, during an EEG study, and significantly more theta relative power, with a higher theta-alpha ratio compared to controls. The fewer alpha waves and more theta activity with an increased theta-alpha ratio represent poor neural processing ability. These results indicate that PCOS even without any comorbidities can present subclinical cognitive impairment.
The performance of verbal fluency, verbal memory, and manual dexterity was shown to be negatively impacted by PCOS [118]. However, no differences were observed in mental rotation, spatial visualization, spatial perception, or perceptual speed. This should indicate that women with PCOS have poor performance on cognitive tasks that usually show a female advantage [118]. Even after a 3-month treatment of anti-androgen plus estradiol resulting in a hormonal reduction of free T levels, PCOS did not exhibit a substantial difference in performance on tests compared to pretreatment scores, apart from verbal fluency [106].
Despite several studies investigating the brain underpinnings of executive functions in PCOS, the cerebral correlates of emotional alterations are still partially undisclosed. Interestingly, the emotional alterations taken into consideration were related to metabolic issues shown by PCOS patients [119]. An association between insulin sensitivity and corticolimbic responses to food pictures was found by comparing high-calorie and low-calorie food pictures using fMRI [120]. The results suggested that this may reflect abnormal brain responses to insulin feedback and contribute to the development and/or perpetuation of obesity in PCOS. Afterward, Van Vugt et al. and Alsaadi and Van Vugt concluded that normal inhibition of corticolimbic brain responses to food pictures during a glucose challenge is compromised in insulin-resistant subjects [121, 122]. They also reported that higher brain responsiveness to food pictures during postprandial hyperinsulinemia might lead to higher non-homeostatic eating and perpetuate obesity in insulin-resistance subjects.
Lastly, according to Lansdown et al., not only was the brain activation in the right orbitofrontal cortex during isometric forearm contraction significantly higher in PCOS but this change was associated with insulin sensitivity [123].
Recent evidence from MRI studies reported structural brain alterations in PCOS. Decreased total brain volume and total gray matter (GM) were observed in PCOS patients [124]. These results were accentuated in obese PCOS when compared with lean PCOS women [125]. PCOS affected by obesity showed decreased GM volume in the caudate nucleus, ventral diencephalon, and hippocampus when compared to healthy controls. On the other hand, lean PCOS patients showed a decreased volume in correspondence of the amygdala. The hormonal changes in PCOS can induce hypertrophy and an increase in the volume of structures such as the pituitary gland. Following this hypothesis, Unlu and colleagues [126] found that the volume of the pituitary gland was significantly increased in a group of 26 PCOS without obesity, and the pituitary volume was positively correlated to the LH/FSH ratio. This result was partially confirmed in a subsequent study that did not observe a correlation between pituitary volume and hormonal values [127].
Other imaging studies showed specific alterations in the white matter (WM) in several brain districts [128, 129]. In a pioneer study, Udiavar and colleagues found decreased mean diffusivity at the level of the genu of the corpus callosum and right cingulum [110]. These alterations, according to the authors, were not related to BMI and pre-PCOS cognitive alterations. However, PCOS showed specific alteration in the white matter involving the insula, and thalamus and extending to the dorsolateral frontal and middle temporal cortex [130]. These WM alterations, positively associated with the levels of free T in PCOS, can suggest the increase in the extracellular space since the Diffusion Weighted images (DWI) were assessed by the apparent diffusion coefficient (ADC). ACD is considered a reliable index to assess the presence of pathological tissue abnormalities in the parenchyma, such as vasogenic edemas [131]. However, in a quantitative study assessing the WM microstructures, no differences were found between PCOS and healthy women [132]. Despite the absence of between-group differences, the authors found a correlation between fractional anisotropy (FA), axial diffusivity (AD), and serum testosterone. In PCOS, insulin resistance is associated with an increase in AD that can be considered an index sensitive to axonal fiber factors [133]. In a recent tractography study, PCOS showed a higher level of structural connectivity than healthy women in the left infundibular region that connects the arcuate nucleus and median eminence [134]. In the same study, the authors assessed the integrity of the hypothalamic membrane metabolism and the glial axonal signaling measuring metabolites, such as N-acetyl aspartate (NAA) and choline (Cho), normalized with the creatine (Cr), using Magnetic Resonance Spectroscopy (MRS). NAA/Cr was significantly higher in the PCOS women than in controls showing a disruption of hypothalamic neuroplasticity. No significant between-group differences were found for Cho/Cr. NAA is a metabolite and an important marker of neuronal functioning [135]. Its variations are usually related to osmoregulatory stress and brain disorders [136].
Functional brain imaging studies highlighted the impact of PCOS on a wider set of brain regions, mainly involving frontal, temporal, and subcortical regions. Functional changes were related to impairments in cognitive functions, such as working and episodic memory and attention. These cognitive impairments were also confirmed by neuropsychological studies that reported decreased performance during the assessment in women with PCOS. Appetitive behaviors and their brain underpinnings were also altered by PCOS. Few and most recent studies investigated the abnormalities in the white and gray matter. Most of them agreed with finding decreased gray matter volume in PCOS. However, hormonal changes observed in PCOS are related to an increased volume of the pituitary gland and neurochemical alteration in patients.
Discussion
PCOS represents one of the most complex and multifaceted disorders and its consequences are evident in many aspects of the life of the patients. PCOS facets are reflected not only in the diagnostic criteria, but also in studies analyzing many aspects of the disease.
Despite the numerous studies that investigated the affected quality of life dimensions, processes and mechanisms underlying PCOS are still partially unknown. Indeed, according to the Bazarganipour model [137], the health-related quality of life in PCOS women is affected by clinical and psychological variables. However, the most prominent clinical variable is hirsutism and infertility which is related to psychological distress, self-esteem, sexual functions, and body image. These variables are in direct and reciprocal relationships, showing the complexity of the psychological alteration found in women with PCOS. As reported in the present review, this phenomenon invades other functions that can affect the quality of life. Although the alteration in body image and the distress that accompanies it, due, for example, to hirsutism and obesity or weight excess, are relatively well characterized, other recent studies showed specific cognitive impairment affecting executive functions and memory. Few studies have investigated cognitive impairment and its cerebral correlates in PCOS patients using in vivo brain imaging techniques. These pioneering studies focused their attention on the substrate of episodic memory and working memory. These specific alterations of memory are related to impaired attention and concentration and are common in cases of stress. Besides, they were previously observed in Post Traumatic Stress Disorder (PTSD) and veterans [138]. In this way, it is possible that memory impairment can be related to psychological distress experienced by PCOS. Nonetheless, psychiatric comorbidities can also affect cognitive functions and cerebral responses in these patients. Most of the patients experience depression, somatization, and bipolar disorder. Mood disorders can affect cognitive functions, such as sustained attention [139] and working memory [140]. Therefore, it is not easy to establish if a specific brain response or cognitive alteration is related to PCOS or is related to comorbidities or stress-related symptoms. However, cognitive impairments, such as impairment of attention or working and episodic memory, need to be taken into consideration for the assessment of the quality of life in these patients.
Similarly, most of the functional brain imaging studies investigated brain alterations underlying working memory and food-related stimuli presentation. No published studies assessed the brain correlates of sexual arousal or desire using fMRI or EEG in women with PCOS, comparing them with normal women or women affected by Hypoactive Sexual Desire Disorder.
Alterations of sexual behavior have been frequently identified in women with PCOS, with decreased levels of satisfaction and desire. The assessment of Quality of Life in these patients should also include the assessment of sexual satisfaction, desire, and orgasm. Therefore, the assessment of QoL should also take into account socio-cultural differences and the context in which the patients lived. For this reason, a mixed approach with an anamnestic interview with neuropsychological and psychological testing is advisable.
Moreover, a few recent studies [99] reported the role played by other hormones, such as GLP1, AMH, leptin, insulin, arginine vasopressin, and oxytocin in PCOS. Since their relevance in metabolic and cardiovascular risk [141] as well as in socio-cognitive functions, oxytocin and arginine vasopressin, in particular, need to be taken into account for future studies. In particular, increased plasma levels of arginine-vasopressin have been found in patients affected by depression [142] and depressive mood is very common in PCOS patients.
Conclusion
Several factors can negatively impact the quality of life of women suffering from PCOS. Most of them are directly related to the clinical condition due to hyperandrogenism and the risk of infertility. Symptoms, such as obesity, hirsutism, acne, and the fear of infertility can have a direct impact on self-esteem and also in sexual function. Metabolic and psychiatric comorbidities can affect the well-being of these patients. Moreover, specific cognitive alterations, such as impairments in attention and working memory, can limit PCOS patients in a series of aspects of daily life.
Data availability
Not applicable.
References
Azziz R (2016) PCOS in 2015: new insights into the genetics of polycystic ovary syndrome. Nat Rev Endocrinol 12(2):74–75. https://doi.org/10.1038/nrendo.2015.230
Witchel SF, Teede HJ, Peña AS (2020) Curtailing PCOS. Pediatr Res 87(2):353–361. https://doi.org/10.1038/s41390-019-0615-1
Wolf WM, Wattick RA, Kinkade ON, Olfert MD (2018) Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health 15(11):2589. https://doi.org/10.3390/ijerph15112589
Chiaffarino F, Cipriani S, Dalmartello M, Ricci E, Esposito G, Fedele F, La Vecchia C, Negri E, Parazzini F (2022) Prevalence of polycystic ovary syndrome in European countries and USA: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 279:159–170. https://doi.org/10.1016/j.ejogrb.2022.10.020
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, International PCOS Network (2018) Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 110(3):364–379. https://doi.org/10.1016/j.fertnstert.2018.05.004
March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ (2010) The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25(2):544–551. https://doi.org/10.1093/humrep/dep399
Schmidt TH, Khanijow K, Cedars MI, Huddleston H, Pasch L, Wang ET, Lee J, Zane LT, Shinkai K (2016) Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol 152(4):391–398. https://doi.org/10.1001/jamadermatol.2015.4498
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF, Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society (2009) The androgen excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91(2):456–488. https://doi.org/10.1016/j.fertnstert.2008.06.035
Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K (2012) Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 97(1):28-38.e25. https://doi.org/10.1016/j.fertnstert.2011.09.024
Haraldstad K, Wahl A, Andenæs R, Andersen JR, Andersen MH, Beisland E, Borge CR, Engebretsen E, Eisemann M, Halvorsrud L et al (2019) A systematic review of quality of life research in medicine and health sciences. Qual Life Res 28(10):2641–2650. https://doi.org/10.1007/s11136-019-02214-9
Costa DS, Mercieca-Bebber R, Rutherford C, Tait MA, King MT (2021) How is quality of life defined and assessed in published research? Qual Life Res 30(8):2109–2121. https://doi.org/10.1007/s11136-021-02826-0
Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC (2006) PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG 113(10):1210–1217. https://doi.org/10.1111/j.1471-0528.2006.01008.x
Zawadzki JK (1992) Diagnostic criteria for polycystic ovary syndrome (a rational approach), Blackwell Scientific, pp 377–384
Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19(1):41–47. https://doi.org/10.1093/humrep/deh098
Johnson T, Kaplan L, Ouyang P (2013) NIH EbMW Report. Bethesda, MD: National Institutes of Health 1.
Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, Kelestimur F, Macut D, Micic D, Pasquali R et al (2014) The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol 171(4):1–29. https://doi.org/10.1530/EJE-14-0253
Blay SL, Aguiar JV, Passos IC (2016) Polycystic ovary syndrome and mental disorders: a systematic review and exploratory meta-analysis. Neuropsychiatr Dis Treat 12:2895–2903. https://doi.org/10.2147/NDT.S91700
Dokras A, Stener-Victorin E, Yildiz BO, Li R, Ottey S, Shah D, Epperson N, Teede H (2018) Androgen Excess- Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril 109(5):888–899. https://doi.org/10.1016/j.fertnstert.2018.01.038
Yin X, Ji Y, Chan CLW, Chan CHY (2021) The mental health of women with polycystic ovary syndrome: a systematic review and meta-analysis. Arch Womens Ment Health 24(1):11–27. https://doi.org/10.1007/s00737-020-01043-x
Showkath N, Sinha M, Ghate JR, Agrawal S, Mandal S, Sinha R (2022) EEG-ERP correlates of cognitive dysfunction in polycystic ovarian syndrome. Ann Neurosci 29(4):225–232. https://doi.org/10.1177/09727531221115318
Månsson M, Holte J, Landin-Wilhelmsen K, Dahlgren E, Johansson A, Landén M (2008) Women with polycystic ovary syndrome are often depressed or anxious—a case-control study. Psychoneuroendocrinology 33(8):1132–1138. https://doi.org/10.1016/j.psyneuen.2008.06.003
Moreira S, Soares E, Tomaz G, Maranhão T, Azevedo G (2010) Síndrome dos ovários policísticos: enfoque psicossocial [Polycystic ovary syndrome: a psychosocial approach]. Acta Med Port 23(2):237–242
Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A (2018) Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine 62(2):318–325. https://doi.org/10.1007/s12020-018-1692-3
Chaudhari AP, Mazumdar K, Mehta PD (2018) Anxiety, depression, and quality of life in women with polycystic ovarian syndrome. Indian J Psychol Med 3:239–246. https://doi.org/10.4103/IJPSYM.IJPSYM_561_17
Beck AT, Epstein N, Brown G, Steer RA (1988) An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 56(6):893–897. https://doi.org/10.1037//0022-006x.56.6.893
Beck AT, Steer RA, Brown G (1996) Beck depression inventory–II. Psychological assessment
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32(1):50–55. https://doi.org/10.1111/j.2044-8341.1959.tb00467.x
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23(1):56–62. https://doi.org/10.1136/jnnp.23.1.56
Dai W, Liu J, Qiu Y, Teng Z, Li S, Huang J, Xiang H, Tang H, Wang B, Chen J, Wu H (2022) Shared postulations between bipolar disorder and polycystic ovary syndrome pathologies. Prog Neuropsychopharmacol Biol Psychiatry 115:110498. https://doi.org/10.1016/j.pnpbp.2021.110498
Kitzinger C, Willmott J (2002) The thief of womanhood’: women’s experience of polycystic ovarian syndrome. Soc Sci Med 54(3):349–361. https://doi.org/10.1016/s0277-9536(01)00034-x
Lee I, Cooney LG, Saini S, Sammel MD, Allison KC, Dokras A (2019) Increased odds of disordered eating in polycystic ovary syndrome: a systematic review and meta-analysis. Eat Weight Disord 24(5):787–797. https://doi.org/10.1007/s40519-018-0533-y
Paganini C, Peterson G, Stavropoulos V, Krug I (2018) The overlap between binge eating behaviors and Polycystic Ovarian Syndrome: an Etiological Integrative Model. Curr Pharm Des 24(9):999–1006. https://doi.org/10.2174/1381612824666171204151209
Thannickal A, Brutocao C, Alsawas M, Morrow A, Zaiem F, Murad MH, Javed Chattha A (2020) Eating, sleeping and sexual function disorders in women with polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Clin Endocrinol (Oxf) 92(4):338–349. https://doi.org/10.1111/cen.14153
Zhang J, Ye J, Tao X, Lu W, Chen X, Liu C (2022) Sleep disturbances, sleep quality, and cardiovascular risk factors in women with polycystic ovary syndrome: systematic review and meta-analysis. Front Endocrinol (Lausanne) 13:971604. https://doi.org/10.3389/fendo.2022.971604
Bambhroliya Z, Sandrugu J, Lowe M, Okunlola O, Raza S, Osasan S, Sethia S, Batool T, Hamid P (2022) Diabetes, polycystic ovarian syndrome, obstructive sleep apnea, and obesity: a systematic review and important emerging themes. Cureus 14(6):e26325. https://doi.org/10.7759/cureus.26325
Kahal H, Kyrou I, Uthman OA, Brown A, Johnson S, Wall PDH, Metcalfe A, Parr DG, Tahrani AA, Randeva HS (2020) The prevalence of obstructive sleep apnoea in women with polycystic ovary syndrome: a systematic review and meta-analysis. Sleep Breath 24(1):339–350. https://doi.org/10.1007/s11325-019-01835-1
Li G, Hu J, Zhang S, Fan W, Wen L, Wang G, Zhang D (2020) Changes in resting-state cerebral activity in women with Polycystic Ovary Syndrome: a Functional MR Imaging Study. Front Endocrinol (Lausanne) 11:603279. https://doi.org/10.3389/fendo.2020.603279
Murphy PJ, Campbell SS (2007) Sex hormones, sleep, and core body temperature in older postmenopausal women. Sleep 30(12):1788–1794. https://doi.org/10.1093/sleep/30.12.1788
Beroukhim G, Esencan E, Seifer DB (2022) Impact of sleep patterns upon female neuroendocrinology and reproductive outcomes: a comprehensive review. Reprod Biol Endocrinol 20(1):16. https://doi.org/10.1186/s12958-022-00889-3
Hashemipour S, Ghorbani A, Khashayar A, Olfati H (2021) Association of sleep quality with insulin resistance in obese or overweight subjects. Sleep Sci 14(1):75–78. https://doi.org/10.5935/1984-0063.20200084
Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP (2001) Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab 86(2):517–520. https://doi.org/10.1210/jcem.86.2.7185
Eisenberg E, Legro RS, Diamond MP, Huang H, O’Brien LM, Smith YR, Coutifaris C, Hansen KR, Santoro N, Zhang H (2021) Sleep habits of women with infertility. J Clin Endocrinol Metab 106(11):e4414–e4426. https://doi.org/10.1210/clinem/dgab474
Moran LJ, Deeks AA, Gibson-Helm ME, Teede HJ (2012) Psychological parameters in the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod 27(7):2082–2088. https://doi.org/10.1093/humrep/des114
Ware JE, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 30(6):473–483
Behboodi Moghadam Z, Fereidooni B, Saffari M, Montazeri A (2018) Measures of health-related quality of life in PCOS women: a systematic review. Int J Womens Health 10:397–408. https://doi.org/10.2147/IJWH.S165794
Bazarganipour F, Ziaei S, Montazeri A, Foroozanfard F, Faghihzadeh S (2013) Iranian version of modified polycystic ovary syndrome health-related quality of Life questionnaire: discriminant and convergent validity. Iran J Reprod Med 11(9):753–760
Coffey S, Bano G, Mason HD (2006) Health-related quality of life in women with polycystic ovary syndrome: a comparison with the general population using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Short Form-36 (SF-36). Gynecol Endocrinol 22(2):80–86. https://doi.org/10.1080/09513590600604541
Jones GL, Benes K, Clark TL, Denham R, Holder MG, Haynes TJ, Mulgrew NC, Shepherd KE, Wilkinson VH, Singh M et al (2004) The Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (PCOSQ): a validation. Hum reprod 19(2):371–377. https://doi.org/10.1093/humrep/deh048
Cronin L, Guyatt G, Griffith L, Wong E, Azziz R, Futterweit W, Cook D, Dunaif A (1998) Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 83(6):1976–1987. https://doi.org/10.1210/jcem.83.6.4990
Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L (2007) Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod 22(8):2279–2286. https://doi.org/10.1093/humrep/dem108
Nasiri-Amiri F, Ramezani Tehrani F, Simbar M, Montazeri A, Mohammadpour RA (2016) Health-related quality of life questionnaire for polycystic ovary syndrome (PCOSQ-50): development and psychometric properties. Qual Life Res 25(7):1791–1801. https://doi.org/10.1007/s11136-016-1232-7
Williams S, Sheffield D, Knibb RC (2018) The Polycystic Ovary Syndrome Quality of Life scale (PCOSQOL): development and preliminary validation. Health Psychol Open 5(2):2055102918788195. https://doi.org/10.1177/2055102918788195
Odhaib SA, Nasiri Amiri F, Altemimi MT, Imran HJ, Alidrisi HA, Mohammed MJ, Mansour AA (2021) Development of the first health-related quality of life questionnaires in Arabic for Women with Polycystic Ovary Syndrome (Part I): the creation and reliability analysis of PCOSQoL-47 and PCOSQoL-42 Questionnaires. Cureus 13(4):e14735. https://doi.org/10.7759/cureus.14735
Bazarganipour F, Ziaei S, Montazeri A, Faghihzadeh S, Frozanfard F (2012) Psychometric properties of the Iranian version of modified polycystic ovary syndrome health-related quality-of-life questionnaire. Hum Reprod 27(9):2729–2736. https://doi.org/10.1093/humrep/des199
Taghavi SA, Bazarganipour F, Montazeri A, Kazemnejad A, Chaman R, Khosravi A (2015) Health-related quality of life in polycystic ovary syndrome patients: a systematic review. Iran J Reprod Med 13(8):473–482
Böttcher B, Fessler S, Friedl F, Toth B, Walter MH, Wildt L, Riedl D (2018) Health-related quality of life in patients with polycystic ovary syndrome: validation of the German PCOSQ-G. Arch Gynecol Obstet 297(4):1027–1035. https://doi.org/10.1007/s00404-017-4623-2
Jedel E, Kowalski J, Stener-Victorin E (2008) Assessment of health-related quality of life: Swedish version of polycystic ovary syndrome questionnaire. Acta Obstet Gynecol Scand 87(12):1329–1335. https://doi.org/10.1080/00016340802444762
Stevanovic D, Bozic-Antic I, Stanojlovic O, Vojnovic Milutinovic D, Bjekic-Macut J, Jancic J, Macut D (2019) Health-related quality of life questionnaire for polycystic ovary syndrome (PCOSQ-50): a psychometric study with the Serbian version. Women Health 59(9):1015–1025. https://doi.org/10.1080/03630242.2019.1587664
Bazarganipour F, Taghavi SA, Montazeri A, Ahmadi F, Chaman R, Khosravi A (2015) The impact of polycystic ovary syndrome on the health-related quality of life: a systematic review and meta-analysis. Iran J Reprod Med 13(2):61–70
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO (2016) Polycystic ovary syndrome. Nat Rev Dis Primers 2:16057. https://doi.org/10.1038/nrdp.2016.57
Hashimoto DM, Schmid J, Martins FM, Fonseca AM, Andrade LH, Kirchengast S, Eggers S (2003) The impact of the weight status on subjective symptomatology of the Polycystic Ovary Syndrome: a cross-cultural comparison between Brazilian and Austrian women. Anthropol Anz 61(3):297–310
Schmid J, Kirchengast S, Vytiska-Binstorfer E, Huber J (2004) Infertility caused by PCOS—health-related quality of life among Austrian and Moslem immigrant women in Austria. Hum Reprod 19(10):2251–2257. https://doi.org/10.1093/humrep/deh432
Bazarganipour F, Ziaei S, Montazeri A, Foroozanfard F, Kazemnejad A, Faghihzadeh S (2013) Predictive factors of health-related quality of life in patients with polycystic ovary syndrome: a structural equation modeling approach. Fertil Steril 100(5):1389–1396. https://doi.org/10.1016/j.fertnstert.2013.06.043
Di Francesco S, Caruso M, Robuffo I, Militello A, Toniato E (2019) The impact of metabolic syndrome and its components on female sexual dysfunction: a narrative mini-review. Curr Urol 12(2):57–63. https://doi.org/10.1159/000489420
Gao Z, Liang Y, Deng W, Qiu P, Li M, Zhou Z (2020) Impact of bariatric surgery on female sexual function in obese patients: a meta-analysis. Obes Surg 30(1):352–364. https://doi.org/10.1007/s11695-019-04240-5
Loh HH, Shahar MA, Loh HS, Yee A (2022) Female sexual dysfunction after bariatric surgery in women with obesity: a systematic review and meta-analysis. Scand J Surg 111(1):14574969211072396. https://doi.org/10.1177/14574969211072395
Mendonça CR, Arruda JT, Noll M, Campoli PMO, Amaral WND (2017) Sexual dysfunction in infertile women: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 215:153–163. https://doi.org/10.1016/j.ejogrb.2017.06.013
Brotto L, Atallah S, Johnson-Agbakwu C, Rosenbaum T, Abdo C, Byers ES, Graham C, Nobre P, Wylie K (2016) Psychological and interpersonal dimensions of sexual function and dysfunction. J Sex Med 13(4):538–571. https://doi.org/10.1016/j.jsxm.2016.01.019
Gonçalves WS, Gherman BR, Abdo CHN, Coutinho ESF, Nardi AE, Appolinario JC (2022) Prevalence of sexual dysfunction in depressive and persistent depressive disorders: a systematic review and meta-analysis. Int J Impot Res 35(4):340–349. https://doi.org/10.1038/s41443-022-00539-7
Kalmbach DA, Kingsberg SA, Ciesla JA (2014) How changes in depression and anxiety symptoms correspond to variations in female sexual response in a nonclinical sample of young women: a daily diary study. J Sex Med 11(12):2915–2927. https://doi.org/10.1111/jsm.12692
Soldati L, Bianchi-Demicheli F, Schockaert P, Köhl J, Bolmont M, Hasler R, Perroud N (2020) Sexual function, sexual dysfunctions, and ADHD: a systematic literature review. J Sex Med 17(9):1653–1664. https://doi.org/10.1016/j.jsxm.2020.03.019
Middleton LS, Kuffel SW, Heiman JR (2008) Effects of experimentally adopted sexual schemas on vaginal response and subjective sexual arousal: a comparison between women with sexual arousal disorder and sexually healthy women. Arch Sex Behav 37(6):950–961. https://doi.org/10.1007/s10508-007-9310-0
Wu T, Zheng Y (2021) Effect of sexual esteem and sexual communication on the relationship between body image and sexual function in Chinese Heterosexual Women. J Sex Med 18(3):474–486. https://doi.org/10.1016/j.jsxm.2020.12.006
van den Brink F, Smeets MA, Hessen DJ, Talens JG, Woertman L (2013) Body satisfaction and sexual health in Dutch female university students. J Sex Res 50(8):786–794. https://doi.org/10.1080/00224499.2012.684250
Seal BN, Meston CM (2020) The impact of body awareness on women’s sexual health: a comprehensive review. Sex Med Rev 8(2):242–255. https://doi.org/10.1016/j.sxmr.2018.03.003
McCool ME, Zuelke A, Theurich MA, Knuettel H, Ricci C, Apfelbacher C (2016) Prevalence of female sexual dysfunction among premenopausal women: a systematic review and meta-analysis of observational studies. Sex Med Rev 4(3):197–212. https://doi.org/10.1016/j.sxmr.2016.03.002
Eftekhar T, Sohrabvand F, Zabandan N, Shariat M, Haghollahi F, Ghahghaei-Nezamabadi A (2014) Sexual dysfunction in patients with polycystic ovary syndrome and its affected domains. Iran J Reprod Med 12(8):539–546
Loh HH, Yee A, Loh HS, Kanagasundram S, Francis B, Lim LL (2020) Sexual dysfunction in polycystic ovary syndrome: a systematic review and meta-analysis. Hormones (Athens) 19(3):413–423. https://doi.org/10.1007/s42000-020-00210-0
Cappelletti M, Wallen K (2016) Increasing women’s sexual desire: the comparative effectiveness of estrogens and androgens. Horm Behav 78:178–193. https://doi.org/10.1016/j.yhbeh.2015.11.003
Basson R, Brotto LA, Petkau AJ, Labrie F (2010) Role of androgens in women’s sexual dysfunction. Menopause 17(5):962–971. https://doi.org/10.1097/gme.0b013e3181d59765
Bianchi VE, Bresciani E, Meanti R, Rizzi L, Omeljaniuk RJ, Torsello A (2021) The role of androgens in women’s health and wellbeing. Pharmacol Res 171:105758. https://doi.org/10.1016/j.phrs.2021.105758
Davis SR, Worsley R, Miller KK, Parish SJ, Santoro N (2016) Androgens and female sexual function and dysfunction-findings from the fourth international consultation of sexual medicine. J Sex Med 13(2):168–178. https://doi.org/10.1016/j.jsxm.2015.12.033
Khera M (2015) Testosterone therapy for female sexual dysfunction. Sex Med Rev 3(3):137–144. https://doi.org/10.1002/smrj.53
Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, Marshall JC (2009) Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls–implications for regulation of pubertal maturation. J Clin Endocrinol Metab 94(7):2360–2366. https://doi.org/10.1210/jc.2008-2606
Krysiak R, Drosdzol-Cop A, Skrzypulec-Plinta V, Okopien B (2016) Sexual function and depressive symptoms in young women with nonclassic congenital adrenal hyperplasia. J Sex Med 13(1):34–39. https://doi.org/10.1016/j.jsxm.2015.11.002
Caruso S, Rugolo S, Agnello C, Romano M, Cianci A (2009) Quality of sexual life in hyperandrogenic women treated with an oral contraceptive containing chlormadinone acetate. J Sex Med 6(12):3376–3384. https://doi.org/10.1111/j.1743-6109.2009.01529.x
Wåhlin-Jacobsen S, Pedersen AT, Kristensen E, Laessøe NC, Lundqvist M, Cohen AS, Hougaard DM, Giraldi A (2015) Is there a correlation between androgens and sexual desire in women? J Sex Med 12(2):358–373. https://doi.org/10.1111/jsm.12774
Rellini AH, Stratton N, Tonani S, Santamaria V, Brambilla E, Nappi RE (2013) Differences in sexual desire between women with clinical versus biochemical signs of hyperandrogenism in polycystic ovarian syndrome. Horm Behav 63(1):65–71. https://doi.org/10.1016/j.yhbeh.2012.10.013
Pastoor H, Timman R, de Klerk C, Bramer M, W, Laan ET, Laven JS, (2018) Sexual function in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biomed Online 37(6):750–760. https://doi.org/10.1016/j.rbmo.2018.09.010
Zhao S, Wang J, Xie Q, Luo L, Zhu Z, Liu Y, Luo J, Zhao Z (2019) Is polycystic ovary syndrome associated with risk of female sexual dysfunction? A systematic review and meta-analysis. Reprod Biomed Online 38(6):979–989. https://doi.org/10.1016/j.rbmo.2018.11.030
Jones GL, Balen AH, Ledger WL (2008) Health-related quality of life in PCOS and related infertility: how can we assess this? Hum Fertil (Camb) 11(3):173–185. https://doi.org/10.1080/14647270802078179
Månsson M, Norström K, Holte J, Landin-Wilhelmsen K, Dahlgren E, Landén M (2011) Sexuality and psychological wellbeing in women with polycystic ovary syndrome compared with healthy controls. Eur J Obstet Gynecol Reprod Biol 155(2):161–165. https://doi.org/10.1016/j.ejogrb.2010.12.012
Janssen OE, Hahn S, Tan S, Benson S, Elsenbruch S (2008) Mood and sexual function in polycystic ovary syndrome. Semin Reprod Med 26(1):45–52. https://doi.org/10.1055/s-2007-992924
Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, Mann K, Schedlowski M, van Halteren WB, Kimmig R, Janssen OE (2006) Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress and sexuality. Hum Reprod 21(7):1925–1934. https://doi.org/10.1093/humrep/del069
Worsley R, Santoro N, Miller KK, Parish SJ, Davis SR (2016) Hormones and female sexual dysfunction: beyond estrogens and androgens-findings from the fourth international consultation on sexual medicine. J Sex Med 13(3):283–290. https://doi.org/10.1016/j.jsxm.2015.12.014
Abdalla MA, Shah N, Deshmukh H, Sahebkar A, Östlundh L, Al-Rifai RH, Atkin SL, Sathyapalan T (2022) Impact of metformin on the clinical and metabolic parameters of women with polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials. Ther Adv Endocrinol Metab 13:20420188221127144. https://doi.org/10.1177/20420188221127142
Cera N, Vargas-Cáceres S, Oliveira C, Monteiro J, Branco D, Pignatelli D, Rebelo S (2021) How relevant is the systemic oxytocin concentration for human sexual behavior? A Systematic Review Sex Med 9(4):100370. https://doi.org/10.1016/j.esxm.2021.100370
Yamamoto S, Noguchi H, Takeda A, Arakaki R, Uchishiba M, Imaizumi J, Minato S, Kamada S, Kagawa T, Yoshida A et al (2022) Changes in endogenous oxytocin levels and the effects of exogenous oxytocin administration on body weight changes and food intake in polycystic ovary syndrome model rats. Int J Mol Sci 23(15):8207. https://doi.org/10.3390/ijms23158207
Chang HH, Chang WH, Chi MH, Peng YC, Huang CC, Yang YK, Chen PS (2019) The OXTR polymorphism stratified the correlation of oxytocin and glucose homeostasis in non-diabetic subjects. Diabetes Metab Syndr Obes 12:2707–2713. https://doi.org/10.2147/DMSO.S226245
Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K (2008) Oxytocin receptor-deficient mice developed late-onset obesity. NeuroReport 19(9):951–955. https://doi.org/10.1097/WNR.0b013e3283021ca9
Amin M, Horst N, Wu R, Gragnoli C (2023) Oxytocin receptor (OXTR) is a risk gene for polycystic ovarian syndrome. Eur Rev Med Pharmacol Sci 27(6):2634–2639. https://doi.org/10.26355/eurrev_202303_31800
Conaglen HM, Conaglen JV (2014) Sexual desire in women presenting for antiandrogen therapy. J Sex Marital Ther 29(4):255–267. https://doi.org/10.1080/00926230390195498
Ozgen Saydam B, Yildiz BO (2021) Polycystic ovary syndrome and brain: an update on structural and functional studies. J Clin Endocrinol Metab 106(2):e430–e441. https://doi.org/10.1210/clinem/dgaa843
Lai W, Li X, Zhu H, Zhu X, Tan H, Feng P, Chen L, Luo C (2020) Plasma luteinizing hormone level affects the brain activity of patients with polycystic ovary syndrome. Psychoneuroendocrinology 112:104535. https://doi.org/10.1016/j.psyneuen.2019.104535
Schattmann L, Sherwin BB (2007) Effects of the pharmacologic manipulation of testosterone on cognitive functioning in women with polycystic ovary syndrome: a randomized, placebo-controlled treatment study. Horm Behav 51(5):579–586. https://doi.org/10.1016/j.yhbeh.2007.02.002
Mehrabadi S, Sadatmahalleh SJ, Kazemnejad A, Moini A (2020) Association of acne, hirsutism, androgen, anxiety, and depression on cognitive performance in polycystic ovary syndrome: a cross-sectional study. Int J Reprod Biomed 18(12):1049–1058. https://doi.org/10.18502/ijrm.v18i12.8026
Sukhapure M, Eggleston K, Douglas K, Fenton A, Frampton C, Porter RJ (2022) Free testosterone is related to aspects of cognitive function in women with and without polycystic ovary syndrome. Arch Womens Ment Health 25(1):87–94. https://doi.org/10.1007/s00737-021-01158-9
Soleman RS, Kreukels BPC, Veltman DJ, Cohen-Kettenis PT, Hompes PGA, Drent ML, Lambalk CB (2016) Does polycystic ovary syndrome affect cognition? A functional magnetic resonance imaging study exploring working memory. Fertil Steril 105(5):1314-1321.e1. https://doi.org/10.1016/j.fertnstert.2016.01.034
Udiawar M, Michael O, Rees A (2014) Reduced cognitive performance and altered white matter microstructure in young insulin-resistant women with polycystic ovary syndrome. Endocrine Abstracts 34.
Koyama MS, O’Connor D, Shehzad Z, Milham MP (2017) Differential contributions of the middle frontal gyrus functional connectivity to literacy and numeracy. Sci Rep 7(1):17548. https://doi.org/10.1038/s41598-017-17702-6
Ishkhanyan B, Michel Lange V, Boye K, Mogensen J, Karabanov A, Hartwigsen G, Siebner H (2020) Anterior and posterior left inferior frontal gyrus contribute to the implementation of grammatical determiners during language production. Front Psychol 11:685. https://doi.org/10.3389/fpsyg.2020.00685
Brewer JA, Garrison KA, Whitfield-Gabrieli S (2013) What about the “Self” is processed in the posterior cingulate cortex? Front Hum Neurosci 7:647. https://doi.org/10.3389/fnhum.2013.00647
Leech R, Sharp DJ (2014) The role of the posterior cingulate cortex in cognition and disease. Brain 137(Pt 1):12–32. https://doi.org/10.1093/brain/awt162
Elmer S (2016) Broca Pars Triangularis Constitutes a “Hub” of the language-control network during simultaneous language translation. Front Hum Neurosci 10:491. https://doi.org/10.3389/fnhum.2016.00491
Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP (2010) Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron 68(1):138–148. https://doi.org/10.1016/j.neuron.2010.09.021
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53(4):695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Schattmann L, Sherwin BB (2007) Testosterone levels and cognitive functioning in women with polycystic ovary syndrome and in healthy young women. Horm Behav 51(5):587–596. https://doi.org/10.1016/j.yhbeh.2007.02.007a
Van Vugt DA, Krzemien A, Alsaadi H, Palerme S, Reid RL (2013) Effect of insulin sensitivity on corticolimbic responses to food picture in women with polycystic ovary syndrome. Obesity (Silver Spring) 21(6):1215–1222. https://doi.org/10.1002/oby.20148
Van Vugt DA, Krzemien A, Alsaadi H, Frank TC, Reid RL (2014) Glucose-induced inhibition of the appetitive brain response to visual food cues in polycystic ovary syndrome patients. Brain res 1558:44–56. https://doi.org/10.1016/j.brainres.2014.02.037
Alsaadi HM, Van Vugt DA (2015) Insulin sensitivity affects corticolimbic brain responses to visual food cues in polycystic ovary syndrome patients. Horm Mol Biol Clin Investig 24(2):101–115. https://doi.org/10.1515/hmbci-2015-0048
Lansdown AJ, Warnert EAH, Sverrisdóttir Y, Wise RG, Rees DA (2019) Regional cerebral activation accompanies sympathoexcitation in women with polycystic ovary syndrome. J Clin Endocrinol Metab 104(9):3614–3623. https://doi.org/10.1210/jc.2019-00065
Chen M, Huang C, Ho H (2023) Decelerated gray matter deterioration with age in young women with PCOS. Fertil Steril 120(4):e226. https://doi.org/10.1016/j.fertnstert.2023.08.645
Ozgen Saydam B, Has AC, Bozdag G, Oguz KK, Yildiz BO (2017) Structural imaging of the brain reveals decreased total brain and total gray matter volumes in obese but not in lean women with polycystic ovary syndrome compared to body mass index-matched counterparts. Gynecol Endocrinol 33(7):519–523. https://doi.org/10.1080/09513590.2017.1295440
Unlu E, Unlu BS, Turamanlar O, Acay MB, Kacar E, Yıldız Y, Verim O, Okur N, Balcik C, Tasgetiren S, Yucel A (2015) Alterations in pituitary gland volume in polycystic ovary syndrome: a structural magnetic resonance imaging study. Clin Imaging 39(3):449–453. https://doi.org/10.1016/j.clinimag.2014.10.002
Bozkurt Koseoglu S, Dinc Elibol F (2018) Does the pituitary gland volume change in polycystic ovary syndrome? Gynecol Obstet Invest 83(5):515–519. https://doi.org/10.1159/000489495
Guoqing Z, Fang S, Lihui D, Bing Y, Qiaoling P, Yingting W, Jinxia L (2016) Cerebral white matter lesions and silent cerebral infarcts in postmenopausal women with polycystic ovary syndrome. Gynecol Endocrinol 32(8):655–658. https://doi.org/10.3109/09513590.2016.1149812
Huddleston HG, Jaswa E, Casaletto K, Neuhaus J, Yaffe K (2022) Polycystic ovary syndrome and differences in brain health at mid-life: results from the cardia cohort. Fertil Steril 118(4):e44–e45. https://doi.org/10.1016/j.fertnstert.2022.08.144
Unlu E, Duran AH, Balcik C, Beker-Acay M, Yildiz Y, Tulmac OB, Unlu BS, Yucel A (2017) Brain diffusion changes in polycystic ovary syndrome. Can Assoc Radiol J 68(4):414–418. https://doi.org/10.1016/j.carj.2017.04.004
Sener RN (2001) Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph 25(4):299–326. https://doi.org/10.1016/s0895-6111(00)00083-5
Rees DA, Udiawar M, Berlot R, Jones DK, O’Sullivan MJ (2016) White matter microstructure and cognitive function in young women with Polycystic Ovary Syndrome. J Clin Endocrinol Metab 101(1):314–323. https://doi.org/10.1210/jc.2015-2318
Winklewski P, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A (2018) Understanding the physiopathology behind axial and radial diffusivity changes-what do we know? Front Neurol 9:92. https://doi.org/10.3389/fneur.2018.00092
Barbotin AL, Mimouni NEH, Kuchcinski G, Lopes R, Viard R, Rasika S, Mazur D, Silva MSB, Simon V, Boursier A et al (2023) Hypothalamic neuroglial plasticity is regulated by anti-Müllerian hormone and disrupted in polycystic ovary syndrome. EBioMedicine 90:104535. https://doi.org/10.1016/j.ebiom.2023.104535
Xu S, Yang J, Shen J (2008) Measuring N-acetylaspartate synthesis in vivo using proton magnetic resonance spectroscopy. J Neurosci Methods 172(1):8–12. https://doi.org/10.1016/j.jneumeth.2008.04.001
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81(2):89–131. https://doi.org/10.1016/j.pneurobio.2006.12.003
Bazarganipour F, Ziaei S, Montazeri A, Foroozanfard F, Kazemnejad A, Faghihzadeh S (2014) Health-related quality of life in patients with polycystic ovary syndrome (PCOS): a model-based study of predictive factors. J Sex Med 11(4):1023–1032. https://doi.org/10.1111/jsm.12405
Isaac CL, Cushway D, Jones GV (2006) Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clin Psychol Rev 26(8):939–955. https://doi.org/10.1016/j.cpr.2005.12.004
Piani MC, Maggioni E, Delvecchio G, Brambilla P (2022) Sustained attention alterations in major depressive disorder: a review of fMRI studies employing Go/No-Go and CPT tasks. J Affect Disord 303:98–113. https://doi.org/10.1016/j.jad.2022.02.003
Xi C, Liu Z, Zeng C, Tan W, Sun F, Yang J, Palaniyappan L (2023) The centrality of working memory networks in differentiating bipolar type I depression from unipolar depression: a task-fMRI study. Can J Psychiatry 68(1):22–32. https://doi.org/10.1177/07067437221078646
Karbek B, Ozbek M, Karakose M, Topaloglu O, Bozkurt NC, Cakır E, Aslan MS, Delibasi T (2014) Copeptin, a surrogate marker for arginine vasopressin, is associated with cardiovascular risk in patients with polycystic ovary syndrome. J Ovarian Res 7:31. https://doi.org/10.1186/1757-2215-7-31
van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, De Wied D (1997) Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology 17(4):284–292. https://doi.org/10.1016/S0893-133X(97)00054-7
Marsh CA, Berent-Spillson A, Love T, Persad CC, Pop-Busui R, Zubieta JK, Smith YR (2013) Functional neuroimaging of emotional processing in women with polycystic ovary syndrome: a case-control pilot study. Fertil Steril 100(1):200–7.e1. https://doi.org/10.1016/j.fertnstert.2013.02.054
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.P., N.C and D.P.; methodology, J.P; software, N.C.; validation, J.P., N.C and D.P; writing—original draft preparation, J.P.; writing—review and editing, N.C. and D.P.; visualization, J.P.; supervision, D.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
D.P. is a member of the editorial board of the Journal of Endocrinological Investigation.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinto, J., Cera, N. & Pignatelli, D. Psychological symptoms and brain activity alterations in women with PCOS and their relation to the reduced quality of life: a narrative review. J Endocrinol Invest (2024). https://doi.org/10.1007/s40618-024-02329-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40618-024-02329-y