Abstract
Background
Cost-of-illness studies in palliative care are of growing interest in health economics. There is no standard methodology to capture direct and non-direct healthcare and non-healthcare expenses incurred by health services, patients and their caregivers in the course of the ambulatory palliative care process.
Objective
We aimed to describe the type of healthcare and non-healthcare expenses incurred by patients with cancer and non-cancer patients and their caregivers for palliative care in ambulatory-based settings and the methodology used to capture the data.
Methods
We conducted a systematic review of studies on the costs of ambulatory-based palliative care in patients with cancer (breast, lung, colorectal) and non-cancer conditions (chronic heart failure, chronic obstructive pulmonary disease, dementia) found in six bibliographic databases (PubMed, EMBASE [via Ovid], Cochrane Database of Systematic Reviews, EconLit, the National Institute for Health Research Health Technology Assessment Database and the National Health Service Economic Evaluation Database at the University of York, and Google Scholar). The studies were published between January 2000 and December 2022. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology for study selection and assessed study quality using the Quality of Health Economic Studies instrument. The study was registered in PROSPERO (CRD42021250086).
Results
Of 1434 identified references, 43 articles met the inclusion criteria. The primary data source was databases. More than half of the articles presented data from public healthcare systems (65.12%) were retrospective (60.47%), and entailed a bottom-up costing analysis (93.2%) made from a healthcare system perspective (53.49%). The sociodemographic characteristics of patients and families/caregivers were similar across the studies. Cost outcomes reports were heterogeneous; almost all of the studies collected data on direct healthcare costs (97.67%). The main driver of costs was inpatient care (55.81%), which increased during the end-of-life period. Nine studies (20.97%) recorded costs due to productivity losses for caregivers and three recorded such costs for patients. Caregiving costs were explored through an opportunity cost analysis in all cases, based on interviews conducted with and questionnaires administered to patients and caregivers, mainly via telephone calls (23.23%).
Conclusions
This systematic review reveals that studies on the costs of ambulatory-based palliative care are increasing. These studies are mostly conducted from a healthcare system perspective, which leaves out costs related to patients’/caregivers’ economic burden. There is a need for prospective studies to assess this financial burden and evaluate, with strong evidence, the interventions and actions designed to improve the quality of life of palliative care patients. Future studies should propose cost calculation approaches using a societal perspective to better estimate the economic burden imposed on patients in ambulatory-based palliative care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ambulatory palliative care costs need to be adequately quantified. The appropriate components to be included and the specific methodology to be used to obtain data on these costs need to be better defined. |
In ambulatory palliative care settings, a significant portion of expenses is transferred to patients/caregivers; the impact of this situation can manifest in several ways. |
As palliative care expenses are shared between the healthcare system and patients/caregivers, a specific methodology for recording and measuring these expenses should be developed. |
1 Introduction
Since the hospice movement started in the UK in 1967, the conceptual framework around palliative care (PC) has changed. Currently, healthcare systems worldwide must care for an older and sicker population receiving effective, long-lasting and expensive therapeutics. The need for universal coverage with an individualised medicine approach to achieve the best health-related quality of life stands in contrast to the scarcity of economic resources available for that purpose.
The idea that PC should be started only when patients with cancer are near death has changed. Palliative care can be initiated to alleviate symptoms in various cancer and non-cancer conditions several months before the patient’s death. Healthcare services and patients have changed, and both curative and palliative interventions, previously restricted to in-hospital settings, can be delivered in ambulatory-based settings such as primary care clinics, hospital outpatient services or even at home (the option most preferred by patients and their families/caregivers [1,2,3,4,5,6]), all depending on the patient’s health status, the system resources, and the health and social network that provides support [7,8,9].
The World Health Organization has defined PC as “an approach that improves the quality of life of patients and their families (adults and children) who are facing problems associated with life-threatening illness. It prevents and relieves suffering through the early identification, correct assessment and treatment of pain and other problems, whether physical, psychosocial, or spiritual” [10]. The European Association of Palliative Care defines PC as “active, total care of patients whose disease is not responsive to curative treatment. Palliative care takes a holistic approach, addressing physical, psychosocial, and spiritual care, including the treatment of pain and other symptoms. Palliative care is interdisciplinary in its approach and encompasses the care of the patient and their family, and should be available in any location, including hospital, hospice, and community …” [11]. These definitions encompass several scenarios for the provision of inpatient and ambulatory care.
Palliative care is now a part of early treatment and is provided in a broad spectrum of situations, not restricted to cancer inpatient settings. The variety of conditions and the care needed by patients have turned PC into a complex intervention for which the record-keeping process is not well defined. The resources consumed and the billing process are not standardised or clear-cut, and consequently our knowledge of healthcare costs related to PC is far from complete.
When PC is delivered as part of inpatient care (in a hospital setting), the billing process is standardised and the cost calculation is relatively straightforward. When PC is provided as an outpatient service or in a primary care or home setting; however, the billing process changes and numerous direct, indirect and non-tangible costs are left out of the final calculation.
In ambulatory scenarios, the fact that PC expenditure is shared by the healthcare system and patients/caregivers may lead to the misconception that PC is less costly. From a health system stakeholder’s perspective, it represents a monetary benefit to the system. However, if viewed from a societal perspective, it becomes evident that the money that is not being invested by the system in inpatient care is being spent by patients and families/caregivers to keep patients comfortable, physically, psychosocially and spiritually, while at home [5, 12,13,14].
When the ambulatory approach is included in the calculations, non-paid out-of-pocket expenditures, loss of productivity of patients and caregivers, time devoted to care, leisure time lost by caregivers, and the psychological and emotional burden placed on patients and caregivers must be factored in and quantified. When all these factors are considered, ambulatory PC may be even more expensive than inpatient PC, depending on the population studied and the analysis performed, but this has not been adequately addressed [15,16,17,18,19]. Palliative care in ambulatory-based settings needs to be appropriately measured.
The three most common cancer conditions in Europe are breast, lung and colorectal neoplasms, which account for more than 25% of cancer cases. Chronic heart failure (CHF) and chronic obstructive pulmonary disease (COPD) are the most frequent non-cancer conditions, accounting for almost 15% of long-standing chronic diseases, [20] and it is estimated that by 2050 dementia (including Alzheimer’s disease [AD]) will rank third in terms of total patient care costs [21]. A large part of the healthcare budget allocated to PC is currently for the management of these pathologies. Still, clear information on the distribution of resources, the costs and the billing process for the services delivered is lacking, which offers a field for research.
Cost-of-illness studies aim to identify, measure, and appraise the healthcare and non-healthcare costs resulting from illness, premature death, or disability due to a condition and its related comorbidities and the associated billing process. Palliative care is an area of medical practice that looks after patients and caregivers in complex health, social, and emotional situations, and studies of costs and billing in this area are lacking. Identifying the most appropriate methodology to follow in economic studies in this area is complex, and a standardised approach has yet to be devised [22,23,24]. The lack of adequate information on costing and billing of the PC services exposes a knowledge gap that needs to be explored. There is thus a need for a systematic literature review (SLR) to describe the most relevant costs in ambulatory-based settings and the most appropriate methodology for appraising the existing data. This review aims to identify, describe and summarise the most common costs of ambulatory PC for patients with cancer and non-cancer patients and their caregivers and the different methodologies used to appraise and calculate them.
2 Methods
2.1 Literature Search
We conducted a SLR following the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology, [25] with a protocol registered in PROSPERO (CRD42021250086). Search terms combined variants of “palliative care”, “cost” and various types of disease. The search strategy included general and Medical Subject Heading terms combined with the Boolean connectors AND OR (Table S1 of the Electronic Supplementary Material [ESM]) and was conducted on PubMed, EMBASE, Cochrane, EconLit, and the National Institute for Health Research Health Technology Assessment Database and the National Health Service Economic Evaluation Database using the Ovid platform. The search was restricted to studies published from 2000 to 2022 that included adult patients. Grey literature was searched in Google Scholar, and article references were hand searched for additional papers.
2.2 Study Selection
We included studies on the costs of PC for patients with various types of cancer (breast, lung or colorectal) and non-cancer patients (mainly those with CHF, COPD or dementia/AD). There was no language restriction. Studies that focused mainly on hospital costs, complete economic evaluations, posters, letters to the editor, case reports and case series were excluded.
To include studies in the SLR, we operationalised the following terms based on other authors’ definitions:
Outpatient/ambulatory services: specialist-level PC services, either as a brief consultation or as a concurrent care model, provided in collaboration with the patient’s primary treating physician (and nurse). The aim is to provide immediate post-discharge follow-up, continuity of care according to plans developed in the hospital, medication reconciliation, and responsiveness to patients’ and family members’ questions and concerns after the patient returns home from the hospital [26].
Inpatient services: those provided in hospitals (intensive care, inpatient care, emergency department visits, ambulance services and day case treatments) [6].
Home-based services: pain management, symptom control and psychosocial support delivered by a specialised trained team. Nurses or physicians provide PC with or without connection to a hospital or hospice. The team also provides psychosocial support to family members [27].
Hospice services: community-based care that is offered, ideally, by a multidisciplinary team that supports patients with advanced disease and their families, [28] and can be provided in a patient’s home setting or in palliative and hospice facilities. Hospice care may be provided to outpatients or inpatients [29].
Caregivers: individuals (e.g. adult children, spouses, parents, friends and neighbours) who provide care that is typically uncompensated and usually at home, which involves significant amounts of time and energy for months or years, and that requires the performance of tasks that may be physically, emotionally, socially or financially demanding [30].
2.3 Data Extraction
A predesigned data collection database was used to extract relevant information from the selected papers: data on general characteristics, design and methodology, clinical data from patients, and sociodemographic information from patients and caregivers, in addition to data on the type of costs recorded and the methods used to measure them. The general conclusions of every study were of interest to establish the utility of the data collected (Table S2 of the ESM). The first screening was conducted by the lead author (AHPB). Each abstract and paper selected was reviewed by two investigators (MTB and CD), and data extraction was performed independently. The decision for inclusion in the review was made by two investigators (AHPB and MTB). Whenever there was a disagreement, the papers were reviewed by a third investigator (CD). Microsoft Excel® was used to summarise and Stata 14.2® to analyse the results from the SLR. After articles that met the inclusion criteria were collected, summary descriptive statistics were used to describe individuals’ demographic and clinical characteristics, the studies’ methodological characteristics and the type of costs analysed. A quantitative analysis of costs was not performed because of the heterogeneity of data units reported; therefore, the results do not show mean unit or annual costs but rather the main types of costs included in the calculations, considering the different settings and types of disease. All descriptive results are expressed in percentages, but the actual number of studies is always indicated to avoid overstating the results, in view of the limited number of studies found.
2.4 Risk of Bias
Study quality, based on the Quality of Health Economic Studies (QHES) grading system [31, 32], was double reviewed and rated by two investigators (AHPB, MTB). The QHES criterion (quality categories [QC]) checklist was constructed and validated to evaluate cost-minimisation, cost-effectiveness and cost-utility analyses. The QHES grading system emphasises appropriate methods, valid and transparent results, and comprehensive reporting of results of each study [31] and has been used previously to evaluate the risk of bias in cost analysis and economic burden studies [33]. In the QHES grading system, items 1, 2, 7, and 12–16 (Fig. 1, grey vertical bars) reflect the extent to which studies reported data in the original publication, and items 3–6 and 8–11 reflect study quality (Fig. 1, blue vertical bars) [32]. The QHES instrument, like other instruments applied to cost studies, such as the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement [34], serves as a checklist for the reporting of items. The more items included, the more complete the information. Hence, the use of the QHES instrument allowed us to assess the quality of reporting of a study and, partially, the quality of the way in which the studies had been conducted. During the QHES criterion validation process, the authors proposed four quality categories as a result of the process of weighting the scores obtained (QC1-QC2-QC3-QC4) and suggested that the higher the category, the greater the reporting of data and the higher the quality of the methodology [31]. We classified every article included in one of these categories to show its overall completeness.
3 Results
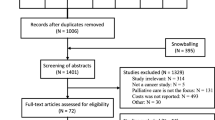
The search identified 1434 papers. After removing duplicates and documents that did not meet the inclusion criteria (1324 in total), and following a hand search of papers’ references, 117 articles were thoroughly read. Of those, 43 studies were ultimately included in the SLR (Fig. 2).
3.1 Risk of Bias Assessment
Only one study was categorised as QC1 and three studies (6.98%) as QC2; 34 studies (79.07%) had scores of between 51 and 75 (QC3), and five studies (11.63%) scored 76 or more (QC4) [Table S3 of the ESM]) (Fig. 3). None of the studies was excluded because of the QC score, and therefore all are included in the final count.
In this SLR, none of the included studies was a “complete” economic evaluation (see Sect. 2.2), and therefore items Q12 and Q13 (8 and 7 points, respectively) were not mandatory in any of the included papers. Few scored points for Q12 [13, 14, 35,36,37,38] and only one scored points for Q13 [37] because it was proposed as a modelling study.
In the case of high scores related to data reporting (Fig. 1, grey bars), the highest number of studies falling into category QC4 were those in which the study objective was clearly reported (Q1) [reported by all except one study [39]], followed by those reporting the method of data abstraction (Q7) [reported by all except [2, 39,40,41,42,43]], those presenting conclusions/recommendations based on study results (Q15) [reported by all except one study [44]] and those disclosing the source of funding (Q16) [reported by all except [1, 3, 7, 35, 39, 40, 42, 45]].
With regard to items relating to study methodology quality (Fig. 1, blue bars), only one study rated as QC1 (Fig. 3, red bar), based on the low number of items reported (14). A subgroup analysis (Q4) was mentioned by only three studies [46], all of which rated as QC3; performance of incremental analysis between alternatives (Q6) was reported only by two studies [1], which rated as QC3 and QC4, respectively. Item Q4 (1 point) is virtually mandatory in the case of a randomised controlled trial, but few of the included studies were randomised controlled trials. Item Q6 has more weight in the QHES (6 points) and is mandatory in the case of complete economic evaluations, but in the case of cost studies there are no specific suggestions in any guidelines. The highest number of studies reporting items relating to methodology (QC4, Fig. 3, green bar) were those reporting the source of variable estimates (Q3) [reported in all studies except [39]]; analytic horizon, outcomes and justification of discount rate (when reported) (Q8) [reported in 37 studies, but not in [5, 15, 39, 40, 42, 45]]; methodology for measurement of costs and estimation of quantities/unit costs (Q9) [reported in 36 studies, but not in [2,3,4, 39, 40, 42, 43]]; primary outcome measure (Q10) [reported in 42 studies, but not in [40]]; and valid health outcomes measures/scales (Q11) [reported in all studies except [39]]. In all cases, the QHES score raised as the completeness of reporting in both domains (data report and study quality) increased (Table S6 of the ESM).
3.2 General Characteristics of Studies
The data collected from the selected studies are from the databases created and the patients/caregivers interviewed from April 1993 [41] to September 2018 [47]. More than half of the studies (32 [69.77%]) were published from 2010 to 2022.
Most of the studies were conducted in the Americas, mainly in North America (the USA and Canada) [25 (58.14%)], and one in Latin America (Argentina). Seven were conducted in Europe (Finland, Germany, Spain, Italy, Greece and Ireland), four in the UK, four in Asia (India, China and Japan), one in Africa (Ethiopia) and one was multinational (USA, Ireland and the UK). The main characteristics of the included studies are summarised in Table S2 of the ESM.
More than half of the studies presented data on exclusively public healthcare programmes in the Americas, Asia and Europe (28 [65.12%]). Public-private services were mentioned in seven studies from Canada [9, 13, 15,16,17,18, 48], one from the USA [49], and one from the UK [47]; one study did not provide data on the health services provider [12]. Solely private care was the modality in two studies from the USA [2, 3], one from Greece [1], one from Italy [46] and one from Ethiopia [50]. Several studies (27 [62.79%]) reported data on patients receiving in-hospital care combined with different types of ambulatory care, and 37.20% (16 studies) reported mainly ambulatory or home-based care (Table 1).
Table 2 describes patients’ and caregivers’ main clinical and sociodemographic characteristics. All the studies included patients with cancer. Twenty-five studies (58.14%) reported cancer as a sole condition in the included patients, and 18 (41.86%) reported data on patients with cancer and patients with other non-oncologic diseases for which PC was provided. Out of all the studies with non-cancer patients requiring PC included as part of the sample, 12 (27.91%) had CHF and COPD, the main non-cancer conditions, and 7 (16.28%) reported dementia/AD. The severity of the conditions and the presence of comorbidities, illnesses unrelated to the one that led to the provision of PC, were also reported (for more details, see Table S2 of the ESM).
The study time frame (follow-up time) was variable, with range from up to a month in ten studies (23.81%) to at least 1 year in 18 studies (41.86%) and more than 2 years in one study [35]. Seven studies reported several follow-up and cost-measuring periods (i.e. repeated measures, related to the type of data analysis) [2, 5, 6, 15, 44, 49, 51]. Mean survival time after starting PC was reported in 12 (27.91%) of the studies, and median survival time was reported in seven studies (16.28%); in all cases, it was less than 1 year (Table 2).
All papers reported data on the patients’ characteristics, and ten (23.26%) reported data related to caregivers. Patient ethnicity was diverse and reported in nine studies; five were from the USA [2, 3, 7, 38, 52], one was from the UK [47], one was from China (Hong Kong) [45] and one was multinational (UK, Ireland, USA) [6]. The caregivers’ ethnicity was not mentioned in any paper.
The clinical professionals in the PC teams assigned to patients were diverse. Some had a specialist physician as a team leader and worked with other physicians, nurses, social workers and other healthcare personnel (e.g. various types of therapists). Others had nurses as team leaders [43]. There was no homogeneity in the composition of the teams (Table S4 of the ESM).
3.3 Cost Methodology
Thirty-three studies (76.74%) were of costs only, ten (23.26%) were analyses of resource use (three cohort and six descriptive studies) and one used a decision model to estimate costs over longer time horizons [37] (Table 3). The predominant cost methodology was bottom-up (88.37%), and the main study design was retrospective (60.47%). The most common perspective for the analysis was that of the national health system (financer) (53.49%), and direct healthcare costs were the most commonly measured and evaluated costs. The follow-up period was reported in all 43 studies and was up to a year in 33 of them (76.74%).
All studies except for one [39] reported data on direct healthcare costs, and 12 (27,6%) reported data on direct non-healthcare costs [6, 9, 13,14,15,16,17,18, 47, 53,54,55]; 12 (27.91%) studies explored direct and indirect costs borne by patients and caregivers; three studies (6.99%) explored patients’ productivity losses [12, 45, 56] and ten (23.26%) caregivers’ productivity losses [9, 12, 13, 15,16,17,18, 45, 55, 56], all from a societal perspective (Table 3). Studies focusing on a societal perspective were mainly prospective.
Data on costs for a 1-year time horizon were collected through questionnaires administered to patients’ caregivers, using the opportunity costs method and collecting data on out-of-pocket expenditures, such as travel expenses, medications needed because of being a caregiver, and time and/or income loss. Only one study [17] measured the direct healthcare costs for carers. No study conducted a regression analysis to analyse the social and economic factors associated with caregivers’ income losses.
The time horizon and its linked data and discount rate were explicitly reported only in one study [57]. A regression analysis to define the factors that determine cost drivers was conducted in almost half of the studies (19 [44.19%]); a sensitivity analysis to test the robustness of the final result was conducted in six studies, but only one reported the type of analysis used (deterministic) [35] [details on regression variables in Table S5 of the ESM].
Twenty studies reported an increase in care intensity and, consequently, an increase in care costs in the end-of-life (EOL) period. Of these, 11 (25.63%) were studies made from the financer perspective (public or private) [4, 5, 40,41,42, 44, 46, 48, 58, 59], seven (16.31%) from the societal perspective [12,13,14,15,16,17, 55] and two (4.66%) from the provider perspective [38, 49]. The periods of time of increased care intensity ranged from 1 to 24 months.
Original (primary) data were reported in five studies (11.65%) [6, 17, 42, 45, 55]. Data from administrative databases were reported in 26 studies (60.58%), and a mixture of data sources was reported in 12 (27.96%) studies.
Of the five studies that used primary data, one study was conducted from a financer perspective [42], three studies from a societal perspective [17, 45, 55] and one study did not report the perspective [6]. Only one study that collected data from administrative data sources applied a societal perspective [50]; the rest (25 [58.25%]) used a financer or provider perspective.
Of 12 studies conducted from a societal perspective, 11 used a questionnaire to collect data [9, 12,13,14,15,16,17,18, 45, 55, 56]. Four of those studies used a validated questionnaire to collect data from patients/caregivers (Ambulatory and Home Care Record [AHCR©]), and those four studies were conducted by the same research team [13, 15,16,17]. Of the remaining studies, three reported using the same non-validated questionnaire [9, 14, 18], and the other mentioned the use of a national-level survey [55]. In the cases where data were collected from patients/caregivers (13 studies), the questionnaire was administered in a face-to-face scenario in two studies [45, 56] and in written form only in one study [12]. A face-to-face interview and a telephone call was the method in four of the studies [9, 14, 18, 55] and a phone call only in the remaining four studies [13, 15,16,17]. There were no data related to the interview in one study [6].
The valuation method for measuring informal care costs (provided by family/caregivers) was the opportunity cost in all ten studies reported; these costs related to diverse caregiving activities (e.g. accompanying patients to appointments, changing dressings, picking up medications [17]), assisting with basic activities of daily living (personal care, eating and drinking, going to the toilet, mobility indoors) and instrumental activities of daily living (taking medications, household and administrative tasks) [55]. Direct questions were used to ascertain time spent in most cases [9, 13,14,15,16,17,18, 55] (Table 3).
3.4 Cost Outcomes
The costs during the follow-up period were variable and collected during periods that ranged from 1 to 24 months; some of the costs were measured over several periods for the same patients and caregivers in order to determine unit cost variations (see above, Table 2). The most common scenario was the multiple-setting scenario (46.51%), followed by the home-based scenario (30.23%). In six studies (13.95%) in which patients’ care transitioned from in-hospital care to home care, data on costs were collected in both stages. Four studies (9.30%) collected data mainly on the ambulatory use of resources [14, 35, 47, 56].
Inpatient care (acute hospitalisation and visits to the emergency department) was found to be the main source of billing in 24 studies (55.81%); of those, the use of resources from more than one setting but with a dominance of hospitalisation costs was seen in 15 studies (34.95%) [3, 6, 12, 37, 38, 41, 43, 44, 48, 51, 55, 57,58,59]. Four studies (9.32%) [14, 35, 47, 56] reported direct costs covered by patients or unpaid caregivers in mainly ambulatory settings, 12 studies (27.96%) [3, 4, 13, 16,17,18, 40, 46, 49, 54, 60, 61] in exclusively home-based settings, and 26 studies (60.58%) in situations where care was provided in the home setting at some point (Table 1). Other drivers of resource use worth mentioning were blood transfusion, mentioned in two studies (4.66%) [1, 46], and non-quantified volunteer support, mentioned in one study (2.33%) [47].
Almost half of the studies (20 [46.51%]) included data on the augmented use of resources and costs of healthcare in the EOL period. In 12 studies (27.96%), the main driver was the use of in-hospital resources; in three (6.99%) — conducted from a societal perspective — it was the unpaid workload taken on by caregivers [13, 16, 17]; in the remaining four (9.32%), the drivers varied. The period of more intense use of resources ranged from 1 to 12 months; the proportion of change in the use of resources was highly variable, and ranged from 37% in one study [12] to 450% in two others [13, 44] and more than 600% in one study [4]. This change was mainly because of hospitalisation. No studies included modelling to estimate costs over longer time horizons.
A regression analysis was conducted in 19 studies (47.5%) to determine the main drivers of costs/expenditures (see Table S5 of the ESM). Because of the distribution of data results, the independent variable was transformed in 13 studies (32.5%). Of the studies analysed with regression models, the most frequently cited cost drivers were hospitalisation/inpatient care, mentioned in ten studies (25%), and costs owing to unpaid caregiving time, mentioned in four studies (10%) [data not shown]. The independent variable used in the models was heterogeneous across studies (Table S5 of the ESM). Only six studies out of 40 included a sensitivity analysis. In all cases, uncertainty was evaluated with a deterministic univariate or multivariate sensitivity analysis.
3.5 Costs Included
Direct healthcare costs covered by the healthcare system were reported in almost all studies (42 [97.67%]). In contrast, direct non-healthcare costs covered by healthcare systems and direct costs covered by the patient were reported in 13 (30.23%) and 12 studies (27.91%), respectively. To manage the heterogeneity of the data reported, the types of costs were assigned to groups according to the setting (outpatient/ambulatory, inpatient, home-based, multiple care settings) and type of disease (cancer and cancer plus other chronic terminal diseases) (Table 4). The settings where the productivity losses of patients and caregivers were recorded varied, without any discernible pattern. Almost half of the studies (18 [45%]) included a mix of patients with different diagnoses, some with cancer and others with non-cancer conditions, as the main trigger for initiating ambulatory-based PC.
In the case of studies using a societal perspective (11 [25.58%]), productivity losses for patients were reported in two studies (6.99%) [12, 45] and productivity losses of caregivers were reported in nine studies (20.97%) [9, 12, 13, 15,16,17,18, 45, 55]. In terms of patient diagnoses, in the same group of studies conducted from a societal perspective, two studies with cancer as the only condition requiring PC reported productivity losses for patients [12, 45]; four studies (9.32%) including both patients with cancer and patients with cancer and other terminal conditions reported productivity losses for caregivers [9, 13, 18, 55] (Table 4).
The reporting of costs by ambulatory-based setting and disease shows that the most common type of cost included was direct healthcare costs (97.67% of studies) (Table 4). Data on costs related to productivity losses for patients or their families/caregivers in ambulatory-based settings are lacking. Data related to patients were collected in an inpatient/home setting in two cases [12, 45], a home-based setting in two cases [16, 17] and an outpatient/ambulatory setting in one case [56]. Productivity loss for caregivers was reported in nine studies (20.97%), four home-based settings [13, 16,17,18], three in hospital/home settings [9, 15, 45] and two in all possible settings [12, 55]; this information was not reported in the exclusively ambulatory setting.
The most commonly included costs are shown in Table 5. Reported as direct healthcare costs, inpatient care (hospitalisation), physician visits and home care were included in more than 70% of the studies. Physician visits, emergency department visits, medication and nurse visits followed these. Among direct non-healthcare costs, out-of-pocket expenditures and informal spiritual care were the most frequently included and measured costs, followed by professional care at home, medications, private transportation and equipment not covered by the national healthcare system. Patient productivity losses were included far less than caregiver productivity losses. Temporary occupational leave and loss of leisure time were the two most commonly included and quantified costs when the societal perspective was used.
4 Discussion
The current path for PC support is the ambulatory-based setting. Some decades ago, PC services were part of the hospital and hospice setting, and ambulatory or home-based care was considered an exceptional form of service from a logistic point of view. However, patients’ and their caregivers’ medical and social needs have evolved over time, driving a transformation in healthcare systems organisation. To determine the costs and the methodology followed by researchers in these areas to capture data, we proposed an SLR.
To evaluate the methodological quality and transparency of data reporting from the studies included in this SLR, we used the QHES grading system (QC) (Figs. 1 and 3, Table S3 of the ESM). During the process, because of the scarcity of studies related only to costs in ambulatory/home-based PC, we unanimously decided to include all the studies that passed the second complete examination during the literature search (Fig. 2); although several had a low QC score (QC1 [39] and QC2 [2, 40, 42]), they were not excluded. The score was used not as a criterion for removing studies from the SLR but as a method to determine the thoroughness of each study. Hence, even the four studies with the lowest scores (QC1 [0–25] and QC2 [>25–50]) were reviewed and their data were included.
In the final analysis, only one study had very low scores (2.33%) and three studies had low scores (2.33%); a majority had high scores (QC3 [79.07%]) and very high scores (QC4 [11.63%]). This was interpreted as evidence that, even with failings, the quality of cost studies is high, although it could be better. When we looked at which QC domain — reporting of data or study of methodological quality — determined the change in scores among the studies, we found that both contributed: scores were higher as the number of items related to reporting and those related to methodology increased (Table S6 of the ESM). We did not find any pattern that would explain a low or high result related to the QC score as a whole.
Other SLRs on PC costs, including both complete and incomplete economic evaluations, have been published in recent decades. We double checked that all studies included in the four SLRs identified were found in our study. No previous SLR has been conducted specifically on the costs of PC.
Gomes et al. [62] published a Cochrane review of 23 studies (from 1978 to 2012) on the effectiveness and cost effectiveness of home PC services, but not specifically on cost data. The results provide evidence of the effectiveness of home-based PC in terms of increasing the patient’s chance of dying at home and reducing the symptom burden for patients, but no significant impact on the caregiver’s grief. Results related to cost effectiveness were not precise, especially in relation to non-oncologic conditions. Data on the effect on (only) the costs of home-based PC, as opposed to usual care, indicated lower expenses in the home-based PC groups, except in one study from 1986, Greer et al. [38]. However, the differences in costs were statistically significant in just one study (also included in this SLR [Brumley et al. [2]]). Gomes et al. do not report more data on significant differences in costs that would enable us to compare our results with theirs.
In 2017, Gardiner et al. [63] published an SLR (1995–2015) whose results proposed the relevant cost components for economic evaluations on PC and approaches for measuring these costs. The paper suggested a framework for determining PC costs rather than describing cost results. This article was identified by our SLR, but was discarded because it failed to meet the inclusion criteria. However, it could help to define some domains and items for the forthcoming questionnaire for cost collection that will follow this publication.
Yadav et al. [64] published an SLR on healthcare costs for patients with cancer, including 16 studies conducted in the USA between 2008 and 2018. They reported costs in different scenarios (inpatient-based, outpatient/inpatient, home-based and multiple settings) and concluded that the provision of PC after a cancer diagnosis is a cost-saving strategy for the healthcare system, especially when comparing inpatient and outpatient strategies. They report that a home-based approach can save on hospital costs, but there were no data on savings with respect to non-hospital costs. In contrast to this SLR, we have stated the perspective adopted by cost studies, extended the number of years included in the SLR and included studies from around the world. Similarly to our review, the Yadav et al. review concluded that the healthcare system can save money when promoting home-based PC.
Gonzalez-Jaramillo et al. [65] published an SLR that calculated the effectiveness of home-based PC in terms of reducing hospital visits and whether home-based care lowered healthcare costs. They concluded that in patients with cancer and non-cancer patients, home-based PC consistently reduced the number of hospital visits and their length, as well as hospitalisation and overall healthcare costs, findings that reinforced our results. The authors mention that the higher costs of outpatient care are offset by the hospital expenses saved. However, as in the case of Yadav et al., the authors do not specify the perspective used in collecting cost data or the amount of expenses transferred from the healthcare system to the patient and family/caregivers.
Currently, ambulatory and home-based care are frequently used daily services. However, they are not used independently from hospitalisation. Outpatient (or ambulatory) and home-based care can be used as the primary setting, but will always be shared with hospitalisation periods. We found that the delivery of ambulatory services is not uniform; only three studies reported mainly ambulatory services: first, a modelling study to explore the use of resources from the beginning of a strong opioid prescription (EOL) [35]; second, a study collecting data on patients in EOL care in urban areas of Canada [14]; and third, a study exploring PC day services in England, Scotland and Northern Ireland [47]. The rest of the studies concerned patients being treated with a predominantly ambulatory care model and variations of admittance to hospital, emergency department or hospice if needed. More than half of the studies referred to costs related to hospitalisation services as the main cost driver (24 studies [55.81%]). Thus, most of the resources used for ambulatory management of patients are linked to hospital care at some point, a finding that supports our previous statement. Our findings also highlight the fact that the ambulatory approach needs to be integrated or coordinated with other healthcare system levels [13, 16, 17] and support the proposal of classifying ambulatory and home-based PC settings under the ambulatory-based PC label, which we considered more precise.
Ambulatory-based PC services are increasing in PC practice for several reasons. The first is requests from patients who prefer to die at home [62]. Such requests have prompted healthcare systems to change and align their outcomes with those of patients [66, 67], offering ambulatory and home-based PC when possible.
The second reason is the limited number and the high cost of hospital beds available in hospital acute-care settings for patients with chronic cancer and non-cancer conditions when they move from active treatment to PC [68]. These beds are mostly needed by acute patients who require inpatient care. Still, even when patients are in better condition and are able to return home, they are considered to have been transferred to home-based hospitalisation and are still regarded as inpatients. These clinical decisions are supported by studies that report home-based care as a safe and good practice with adequate clinical outcomes and lower costs [13, 69]. Similar studies of ambulatory-based PC are lacking.
The third and no less important reason is billing. The ambulatory approach has been used broadly in elective surgery scenarios. Large studies of acute ambulatory surgery programmes reveal fewer expenses after medium- and high-complexity surgical procedures (e.g. hernia repair, laparoscopic cholecystectomy, laparoscopic appendicectomy, thyroidectomy, total shoulder, hip or knee replacement) [70, 71]. Even some acute medical situations (e.g. pneumonia) can be stabilised during a short inpatient stay, after which the patient is sent home to continue and finish treatment [72]. Because of their short duration, these acute services are provided by paid healthcare personnel who assist the patient at home, allowing both the patient and family to be well assisted [16, 17], a similar approach to PC.
For the reasons mentioned above, ambulatory PC services are being implemented in various healthcare systems worldwide [68]. These PC programmes complement, rather than replace, classical hospital or hospice-based PC services, with excellent economic results from the point of view of healthcare providers: it is far less expensive to deliver this EOL care modality to the patient at home rather than at the hospital. However, what needs to be recognised is that when a PC patient is moved to home-based management, the burden and most of the costs are transferred to patients and their caregivers/families. The indirect costs of productivity loss, out-of-pocket expenditures, loss of leisure time and burn-out of caregivers, for example, are not currently being quantified. Conceptually, ambulatory PC represents a shift of the economic burden from the provider to the patient without substantial support from health systems for patients’ families [9, 12,13,14, 18, 51, 64]. This shifting leaves out of the equation the non-tangible healthcare costs transferred to the family, in particular the contribution made by caregivers [12, 13, 64].
In the inclusion criteria, we proposed to include studies involving patients with breast, colon and pulmonary malignancies, CHF and COPD. These criteria were based on disease prevalence [20, 73]. However, during the screening, we detected two studies conducted with a different sample — patients with haematological neoplasms [1, 46]. We included them in the review to explore the cost approach in these oncologic conditions. We saw that ambulatory PC for these patients was based mainly on transfusions, and the reports barely mentioned the role of caregivers [38] or the burden that the care of patients implies. We also found more studies than expected that reported data on the costs of PC for patients with dementia/AD, and although the PC path was not very clear in those reports, we decided to mention them and examine and include their data [4, 8, 41, 46, 58, 59, 69] (Table 4). What we found reveals that the organisation and the delivery of PC may differ depending on the disease; we also found that our inclusion criteria and the diseases considered may have been too narrow and may have limited our results, making it difficult to extrapolate them. Still, the funnel approach is necessary to deepen the data and to provide more specific results.
The included studies reported cost results using heterogeneous outcome/measure units such as overall cost, mean cost, cost per patient, total cost, average cost and cost per week. Lack of transparency in calculating the different costs and the definition of some types of costs did not facilitate pooling or quantitative summarisation of cost data or the development of a meta-analysis (not a primary aim of this SLR). We suspected this would be the case, based on other studies that mentioned the wide variety of reporting unit costs. Moreover, the appropriate unit for measuring PC in the early stages of cancer or in the case of non-oncological conditions could be annual cost, but as soon as the patients’ health status changes because of the disease’s progression, a more appropriate unit would probably be monthly or weekly costs. Better data pooling and perhaps a meta-analysis could be conducted in the future if more homogeneous methods of reporting unit cost results/outcomes are developed [74].
The follow-up time was variable and diverse, and ranged from less than a month to more than a year, and in some studies, several time periods were examined [2, 5, 15, 37, 41]. We did not find any relationship between follow-up time and diagnosis in the included studies, nor were the criteria for deciding the follow-up time clear in most of them, although there were seven studies that planned a repeated-measures analysis [2, 5, 15, 37, 41, 42].
The follow-up time has cost consequences. Clinically, survival rates of patients with non-cancer conditions included in a PC programme are higher than those of patients with cancer receiving the same PC modality. However, all the evidence in terms of the economic burden or costs of PC patients has been calculated by averaging data on patients with non-cancer diagnoses with those of patients with cancer, and no independent estimations linked to the condition that triggers the PC have been done as yet.
Patients with end-stage COPD, CHF or AD can experience acute episodes of deterioration of their condition, but in most cases, treatment of such deterioration can rapidly ameliorate patients’ health status. Such an outcome is not frequently seen in patients with cancer, in which a severe deterioration in their clinical condition, once the last line of oncological treatment has been completed, invariably progresses to the patients’ death. Thus, the survival expectancy that would define a follow-up time can vary from a few weeks in terminal lung or breast cancer to several years in the case of CHF, COPD or AD.
Calculating a mean or average cost for PC patients and combining patients with a cancer diagnosis and patients with a non-cancer diagnosis is probably an inaccurate method to obtain cost data because of the different evolutions of illnesses requiring PC and the differences in survival rates. The scarcity of cost studies relating exclusively to PC in non-cancer patients, comparing the costs of PC programmes for patients with these different conditions, is remarkable (the initial search included only one complete economic evaluation on CHF [75], which was excluded because it failed to meet the inclusion criteria). There is therefore an urgent need for such studies.
From the cost perspective, we could not find clarity related to the time horizons used for the cost calculations. All studies reported a follow-up period, but the time horizon was not explicitly mentioned. These two points should be kept in mind when planning future research, and both a follow-up time in relation to the clinical outcome and a time horizon concerning cost calculation should be defined. New approaches might have to be created to consolidate PC costs, as suggested by other authors concerning quality-adjusted life-years in a cost-utility analysis for PC [74].
Nineteen studies reported increased use of resources during the EOL period. Among these studies, the main source of augmented costs was inpatient care in studies made from a health system perspective (52%) [5, 34, 36, 38, 41, 44, 54,55,56,57]. When studies were made from a societal perspective, the outcome was not too different; in more than half of such studies, the increase in the use of resources was owing to inpatient care provided at some point [12, 14, 15, 51] and in the rest it was related to care provided by unpaid caregivers [13, 16, 17]. This finding confirms what we previously pointed out: hospitalisation is the main driver of patients’ care costs, even in ambulatory-based PC [9, 12, 14, 15, 18, 45, 51].
Most cost studies that have applied a healthcare system perspective have concluded that using ambulatory PC services as an alternative to hospital-based care diminishes costs for the healthcare system and, therefore, is an efficient alternative. In contrast, all studies applying a societal perspective reveal that ambulatory services are costlier and less efficient than inpatient hospital care [9, 12,13,14,15,16,17,18, 39, 45, 51, 52]. The economic burden borne by family and informal caregivers is explained by the unpaid costs of productivity and leisure time loss. Thus, although the societal perspective entails challenges in terms of approaching patients and caregivers, it should be considered the ideal for evaluating PC services in an ambulatory-based setting (i.e. one that combines hospitalisation and any type of ambulatory care) in order to include economic impacts that are frequently not considered in PC cost studies but that are relevant from an efficiency point of view [13, 16, 17].
This SLR identified the most common costs related to ambulatory-based PC for patients with cancer or non-cancer terminal illnesses. Direct healthcare costs in home-based or multiple care settings have been those most analysed and evaluated, inpatient care (hospitalisation), physician visits and home care being the most common costs included to measure the economic burden of PC. It is worrying that little evidence exists regarding productivity losses for patients receiving PC and their family members or caregivers, especially for patients in an outpatient/ambulatory setting.
The need for ambulatory PC is clear. It is desired by both patients and the healthcare system, and the changing scenario of healthcare makes it possible to provide PC in an ambulatory setting. However, adequate implementation of ambulatory PC has a cost that the healthcare system should bear in partnership with society, and society should receive support for being part of the healthcare team [14, 15, 18].
5 Conclusions
This SLR reveals that studies on the costs of ambulatory PC are increasing. These studies are mostly conducted from a healthcare system perspective, which leaves out costs related to patients’/caregivers’ economic burden; these costs are mainly driven by productivity and leisure time losses. The available evidence shows a lack of studies that measure and evaluate average patient and caregiver expenses, and there is therefore a need for better evidence on the scale and patterns of costs.
Future studies should preferentially propose cost calculation approaches using societal and patient perspectives to better estimate the economic burden imposed on patients and caregivers in ambulatory-based PC. There is a need for prospective studies to calculate this financial burden more precisely and evaluate, with better quality evidence, interventions and actions designed to improve the quality of life of PC patients. Based on the evidence found, productivity losses for PC patients and their carers have been the least studied cost impact, so future research should endeavour to measure and economically quantify such losses.
The results reported in this SLR should serve as guidance for future cost collection studies and questionnaires in ambulatory-based settings from any perspective, but especially those designed to measure and evaluate the costs from a societal perspective, including the carer’s economic burden. The methodology should include a clear plan for collecting administrative data based on its primary structure and organisation and a well-structured, respectful and comprehensive approach to the collection of data from patients and caregivers. An effort to standardise a methodology would be desirable in order to produce more homogeneous biomedical literature on this subject.
References
Tzala S, Lord J, Ziras N, Repousis P, Potamianou A, Tzala E. Cost of home palliative care compared with conventional hospital care for patients with haematological cancers in Greece. Eur J Health Econ. 2005;6:102–6.
Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55:993–1000.
Enguidanos S, Cherin D, Brumley R. Home-based palliative care study: site of death, and costs of medical care for patients with congestive heart failure, chronic obstructive pulmonary disease, and cancer. J Soc Work End Life Palliat Care. 2005;1:37–56.
Cassel JB, Kerr KM, McClish DK, Skoro N, Johnson S, Wanke C, et al. Effect of a home-based palliative care program on healthcare use and costs. J Am Geriatr Soc. 2016;64:2288–95.
Li Z, Pan Z, Zhang L, He R, Jiang S, Xu C, et al. End-of-life cost and its determinants for cancer patients in urban China: a population-based retrospective study. BMJ Open. 2019;9: e026309.
Yi D, Johnston BM, Ryan K, Daveson BA, Meier DE, Smith M, et al. Drivers of care costs and quality in the last 3 months of life among older people receiving palliative care: a multinational mortality follow-back survey across England, Ireland and the United States. Palliat Med. 2020;34:513–23. https://doi.org/10.1177/0269216319896745.
Wang H, Qiu F, Boilesen E, Nayar P, Lander L, Watkins K, et al. Rural-urban differences in costs of end-of-life care for elderly cancer patients in the United States. J Rural Health. 2016;32:353–62.
Terada T, Nakamura K, Seino K, Kizuki M, Inase N. Cost of shifting from healthcare to long-term care in later life across major diseases: analysis of end-of-life care during the last 24 months of life. J Rural Med. 2018;13:40–7.
Dumont S, Jacobs P, Turcotte V, Turcotte S, Johnston G. Palliative care costs in Canada: a descriptive comparison of studies of urban and rural patients near end of life. Palliat Med. 2015;29:908–17.
World Health Organization. WHO definition of palliative care. 2013. Available from: http://www.who.int/cancer/palliative/definition/en. [Accessed 17 Nov 2023].
European Association for Palliative Care. 2020. Available from: https://www.eapcnet.eu/. [Accessed 28 Feb 2020].
Haltia O, Färkkilä N, Roine RP, Sintonen H, Taari K, Hänninen J, et al. The indirect costs of palliative care in end-stage cancer: a real-life longitudinal register- and questionnaire-based study. Palliat Med. 2018;32:493–9. https://doi.org/10.1177/0269216317729789.
Guerriere DN, Zagorski B, Fassbender K, Masucci L, Librach L, Coyte PC. Cost variations in ambulatory and home-based palliative care. Palliat Med. 2010;24:523–32.
Dumont S, Jacobs P, Turcotte V, Anderson D, Harel F. The trajectory of palliative care costs over the last 5 months of life: a Canadian longitudinal study. Palliat Med. 2010;24:630–40.
Yu M, Guerriere DN, Coyte PC. Societal costs of home and hospital end-of-life care for palliative care patients in Ontario, Canada. Health Soc Care Community. 2015;23:605–18.
Chai H, Guerriere DN, Zagorski B, Coyte PC. The magnitude, share and determinants of unpaid care costs for home-based palliative care service provision in Toronto, Canada. Health Soc Care Community. 2014;22:30–9.
Chai H, Guerriere DN, Zagorski B, Kennedy J, Coyte PC. The size, share, and predictors of publicly financed healthcare costs in the home setting over the palliative care trajectory: a prospective study. J Palliat Care. 2013;29:154–62. https://doi.org/10.1177/082585971302900304.
Dumont S, Jacobs P, Turcotte V, Turcotte S, Johnston G. Distribution and sharing of palliative care costs in rural areas of Canada. J Palliat Care. 2014;30:90–8. https://doi.org/10.1177/082585971403000204.
Jacobs P, Dumont S, Turcotte V, Anderson D. Evaluating the economic loss of caregiving for palliative care patients. J Palliat Care. 2011;27:210–5. https://doi.org/10.1177/082585971102700305.
Anguita Sánchez M, Crespo Leiro MG, De Teresa GE, Jiménez Navarro M, Alonso-Pulpón L, Muñiz GJ. Prevalencia de la insuficiencia cardiaca en la población general Española mayor de 45 años. Estudio PRICE Revista Espanola de Cardiologia. 2008;61:1041–9.
Colucci L, Bosco M, Fasanaro AM, Gaeta GL, Ricci G, Amenta F. Alzheimer’s disease costs: what we know and what we should take into account. J Alzheimers Dis. 2014;42:1311–24.
Guerriere DN, Coyte PC. The ambulatory and home care record: a methodological framework for economic analyses in end-of-life care. J Aging Res. 2011;2011: 374237.
Gardiner C, Ingleton C, Ryan T, Ward S, Gott M, Gomersall JS, et al. What cost components are relevant for economic evaluations of palliative care, and what approaches are used to measure these costs? A systematic review. Palliat Med. 2015;31:170–8. https://doi.org/10.1177/0269216317729789.
Gardiner C, McDermott C, Hulme C. Costs of Family Caregiving in Palliative Care (COFAC) questionnaire: development and piloting of a new survey tool. BMJ Support Palliat care. 2019;9:300–6.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6: e1000097.
Meier DE, Beresford L. Outpatient clinics are a new frontier for palliative care. J Palliat Med. 2008;11:823–8.
Nordly M, Vadstrup ES, Sjogren P, Kurita GP. Home-based specialized palliative care in patients with advanced cancer: a systematic review. Palliat Support Care. 2016;14:713–24.
Currow DC, Agar MR, Phillips JL. Role of hospice care at the end of life for people with cancer. J Clin Oncol. 2020;38:937–43. https://doi.org/10.1200/JCO.18.02235.
Frasca M, Galvin A, Raherison C, Soubeyran P, Burucoa B, Bellera C, et al. Palliative versus hospice care in patients with cancer: a systematic review. BMJ Support Palliat Care. 2021;11:188–99.
Biegel DE, Sales ESR. Family caregiving in chronic illness: Alzheimer’s disease, cancer, heart disease, mental illness, and stroke. Thousand Oaks: Sage Publications; 1991.
Chiou CF, Hay JW, Wallace JF, Bloom BS, Neumann PJ, Sullivan SD, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41:32–44.
Ofman JJ, Sullivan SD, Neumann PJ, Chiou CF, Henning JM, Wade SW, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9:53–61.
Norum J, Nieder C. Treatments for metastatic prostate cancer (mPC): a review of costing evidence. Pharmacoeconomics. 2017;35:1223–36.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25:3–9. https://doi.org/10.1016/j.jval.2021.11.1351.
Guest JF, Ruiz FJ, Greener MJ, Trotman IF. Palliative care treatment patterns and associated costs of healthcare resource use for specific advanced cancer patients in the UK. Eur J Cancer Care. 2006;15:65–73.
Walker H, Anderson M, Farahati F, Howell D, Librach SL, Husain A, et al. Resource use and costs of end of life/palliative care: Ontario adult cancer patients dying during 2002–2003. J Palliat Care. 2011;27:79–88.
McBride T, Morton A, Nichols A, Van Stolk C. Comparing the costs of alternative models of end-of-life care. J Palliat Care. 2011;27:126–33. https://doi.org/10.1177/082585971102700208.
Greer JA, Tramontano AC, McMahon PM, Pirl WF, Jackson VA, El-Jawahri A, et al. Cost analysis of a randomized trial of early palliative care in patients with metastatic nonsmall-cell lung cancer. J Palliat Med. 2016;19:842–8.
Wenk R, Bertolino M, Pussetto J. Direct medical costs of an Argentinean domiciliary palliative care model. J Pain Symptom Manage. 2000;20:162–5.
Serra-Prat M, Gallo P, Picaza JM. Home palliative care as a cost-saving alternative: evidence from Catalonia. Palliat Med. 2001;15:271–8.
Fassbender K, Fainsinger R, Brenneis C, Brown P, Braun T, Jacobs P. Utilization and costs of the introduction of system-wide palliative care in Alberta, 1993–2000. Palliat Med. 2005;19:513–20.
Gómez-Batiste X, Tuca A, Corrales E, Porta-Sales J, Amor M, Espinosa J, et al. Resource consumption and costs of palliative care services in Spain: a multicenter prospective study. J Pain Symptom Manage. 2006;31:522–32.
Yosick L, Crook RE, Gatto M, Maxwell TL, Duncan I, Ahmed T, et al. Effects of a population health community-based palliative care program on cost and utilization. J Palliat Med. 2019;22:1075–81.
Hollander MJ. Costs of end-of-life care: findings from the province of Saskatchewan. Healthc Q. 2009;12:50–8.
Chan ATC, Jacobs P, Yeo W, Lai M, Hazlett CB, Mok TSK, et al. The cost of palliative care for hepatocellular carcinoma in Hong Kong. Pharmacoeconomics. 2001;19:947–53.
Cartoni C, Brunetti GA, D’Elia GM, Breccia M, Niscola P, Marini MG, et al. Cost analysis of a domiciliary program of supportive and palliative care for patients with hematologic malignancies. Haematologica. 2007;92:666–73.
Mitchell PM, Coast J, Myring G, Ricciardi F, Vickerstaff V, Jones L, et al. Exploring the costs, consequences and efficiency of three types of palliative care day services in the UK: a pragmatic before-and-after descriptive cohort study. BMC Palliat Care. 2020;19:1–9.
Cheung MC, Earle CC, Rangrej J, Ho TH, Liu N, Barbera L, et al. Impact of aggressive management and palliative care on cancer costs in the final month of life. Cancer. 2015;121:3307–15.
Kerr CW, Donohue KA, Tangeman JC, Serehali AM, Knodel SM, Grant PC, et al. Cost savings and enhanced hospice enrollment with a home-based palliative care program implemented as a hospice–private payer partnership. J Palliat Med. 2014;17:1328–35.
Reid EA, Abathun E, Diribi J, Mamo Y, Wondemagegnhu T, Hall P, et al. Early palliative care in newly diagnosed cancer in Ethiopia: feasibility randomised controlled trial and cost analysis. BMJ Support Palliat Care. 2022. https://doi.org/10.1136/spcare-2022-003996.
Look Hong NJ, Liu N, Wright FC, MacKinnon M, Seung SJ, Earle CC, et al. Assessing the impact of early identification of patients appropriate for palliative care on resource use and costs in the final month of life. JCO Oncol Pract. 2020;16:e688-702.
May P, Garrido MM, Cassel JB, et al. Palliative care teams’ Cost-saving effect is larger for cancer patients with higher numbers of comorbidities. Health Aff (Millwood). 2016;35:44–53.
Dzingina MD, Reilly CC, Bausewein C, Jolley CJ, Moxham J, McCrone P, et al. Variations in the cost of formal and informal health care for patients with advanced chronic disease and refractory breathlessness: a cross-sectional secondary analysis. Palliat Med. 2017;31:369–77.
Seow H, Salam-White L, Bainbridge D. Community-based specialist palliative care teams and health system costs at end of life: a retrospective matched cohort study. CMAJ Open. 2019;7:E73-80.
Brick A, Smith S, Normand C, O’Hara S, Droog E, Tyrrell E, et al. Costs of formal and informal care in the last year of life for patients in receipt of specialist palliative care. Palliat Med. 2017;31:356–68. https://doi.org/10.1177/0269216316686277.
Harsvardhan R, Arora T, Singh S, Lal P. Cost analysis on total cost incurred (including out-of-pocket expenditure and social cost) during palliative care in cases of head-and-neck cancer at a government regional cancer centre in North India. Indian J Palliat Care. 2022;28:419–27.
Emmert M, Pohl-Dernick K, Wein A, Dörje F, Merkel S, Boxberger F, et al. Palliative treatment of colorectal cancer in Germany: cost of care and quality of life. Eur J Health Econ. 2013;14:629–38.
Bremner KE, Krahn MD, Warren JL, Hoch JS, Barrett MJ, Liu N, et al. An international comparison of costs of end-of-life care for advanced lung cancer patients using health administrative data. Palliat Med. 2015;29:918–28.
Huo J, Hong Y-R, Turner K, Diaby V, Chen C, Bian J, et al. Timing, costs, and survival outcome of specialty palliative care in Medicare beneficiaries with metastatic non-small-cell lung cancer. JCO Oncol Pract. 2020;16:e1532–42. https://doi.org/10.1200/OP.20.00298.
Klinger CA, Howell D, Marshall D, Zakus D, Brazil K, Deber RB. Resource utilization and cost analyses of home-based palliative care service provision: the Niagara West End-of-Life Shared-Care Project. Palliat Med. 2013;27:115–22.
Chen CY, Naessens JM, Takahashi PY, McCoy RG, Borah BJ, Borkenhagen LS, et al. Improving value of care for older adults with advanced medical illness and functional decline: cost analyses of a home-based palliative care program. J Pain Symptom Manage. 2018;56:928–35. https://doi.org/10.1016/j.jpainsymman.2018.08.015.
Gomes B, Calanzani N, Curiale V, Mccrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013;2013(6): CD007760.
Gardiner C, Ingleton C, Ryan T, Ward S, Gott M. What cost components are relevant for economic evaluations of palliative care, and what approaches are used to measure these costs? A systematic review. Palliat Med. 2017;31:323–37.
Yadav S, Heller IW, Schaefer N, Salloum RG, Kittelson SM, Wilkie DJ, et al. The health care cost of palliative care for cancer patients: a systematic review. Support Care Cancer. 2020;28:4561–73.
Gonzalez-Jaramillo V, Fuhrer V, Gonzalez-Jaramillo N, Kopp-Heim D, Eychmüller S, Maessen M. Impact of home-based palliative care on health care costs and hospital use: a systematic review. Palliat Support Care. 2021;19:474–87.
Porter I, Gonçalves-Bradley D, Ricci-Cabello I, Gibbons C, Gangannagaripalli J, Fitzpatrick R, et al. Framework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunities. J Comp Eff Res. 2016;5:507–19.
Saenger P, Federman AD, DeCherrie LV, Lubetsky S, Catalan E, Leff B, et al. Choosing inpatient vs home treatment: why patients accept or decline hospital at home. J Am Geriatr Soc. 2020;68:1579–83.
Rimar A, Friedman MI, Quinteros MG, Gooch RA, Masick KD, DaCosta N, et al. Changing the care paradigm for patients: advanced illness beds care model. Am J Hosp Palliat Med. 2021;38:1336–41.
Gardiner C, Robinson J, Connolly M, Hulme C, Kang K, Rowland C, et al. Equity and the financial costs of informal caregiving in palliative care: a critical debate. BMC Palliat Care. 2020;19:1–7.
Friedlander DF, Krimphove MJ, Cole AP, Marchese M, Lipsitz SR, Weissman JS, et al. Where is the value in ambulatory versus inpatient surgery? Ann Surg. 2021;273:909–16.
Pollock M, Somerville L, Firth A, Lanting B. Outpatient total hip arthroplasty, total knee arthroplasty, and unicompartmental knee arthroplasty: a systematic review of the literature. JBJS Rev. 2016;4:1–15.
Butt S, Swiatlo E. Treatment of community-acquired pneumonia in an ambulatory setting. Am J Med. 2011;124:297–300.
International Agency for Research on Cancer, World Health Organization. Global Cancer Observatory. 2022. https://gco.iarc.fr/. [Accessed 5 Nov 2022].
Hughes J. Palliative care and the QALY problem. Health Care Anal. 2005;13:289–301.
Sahlen KG, Boman K, Brännström M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: based on a randomized controlled trial. Palliat Med. 2016;30:296–302.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Acknowledgements
Universitat de Barcelona supports Open Access publishing through the CRUE-CSIC Agreement.
Conflicts of Interest/Competing Interests
Ana Helena Perea-Bello, Marta Trapero-Bertran and Christian Dürsteler have no conflicts of interest that are directly relevant to the content of this article. Disclosure forms provided by the authors are available upon request.
Ethics Approval
This SLR is part of a project approved by the following ethical research committees: Comité de Ética de la Investigación con Medicamentos del Hospital Clínic de Barcelona, 8 October 2021/Reg. HCB/2021/0986; Comitè d’ètica d’Investigació amb Medicaments, IDIAPJordiGol, 23 February 2022/Codi CEIm: 22/006-P; and registered in: PROSPERO, ID: CRD42021250086 (created 4 April 2021) and ClinicalTrials.gov ID: NCT05412784 (created 9 June 2022).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The final dataset is available on request.
Code Availability
Not applicable.
Authors’ Contributions
All authors were involved in the conception and planning of the SLR. AHPB conducted the literature search, and AHPB, MTB and CD performed the screening. AHPB interpreted and synthesised the review results and wrote the paper’s first draft. MTB and CD contributed to the critical review, revisions and final draft corrections. All authors approved the final submitted version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Perea-Bello, A.H., Trapero-Bertran, M. & Dürsteler, C. Palliative Care Costs in Different Ambulatory-Based Settings: A Systematic Review. PharmacoEconomics 42, 301–318 (2024). https://doi.org/10.1007/s40273-023-01336-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01336-w