Abstract
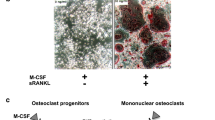
Narlumosbart (津立生) is a recombinant, fully human, anti-receptor activator of nuclear factor kappa-Β ligand (RANKL) IgG4 monoclonal antibody being developed by CSPC Pharmaceutical and its wholly owned subsidiary Shanghai Jinmante Biotechnology for the treatment of giant cell tumour of bone (GCTB), bone metastases from solid tumours and osteoporosis. The RANK/RANKL signalling pathway plays a pivotal role in osteoclastogenesis and in the pathogenesis of GCTB. Narlumosbart specifically binds to RANKL and blocks the interaction of RANKL with RANK, thus inhibiting osteoclastogenesis and bone resorption by osteoclasts. In September 2023, narlumosbart received conditional first approval in China for the treatment of adults with GCTB that is unresectable or when surgical resection would result in severe functional disability. Clinical studies of narlumosbart for bone metastases, postmenopausal osteoporosis and glucocorticoid-induced osteoporosis are underway in China. This article summarizes the milestones in the development of narlumosbart leading to this first approval for the treatment of adults with GCTB.
Similar content being viewed by others
References
Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol. 2000;156(3):761–7.
Dufresne A, Derbel O, Cassier P, et al. Giant-cell tumor of bone, anti-RANKL therapy. Bonekey Rep. 2012;1:149.
Atkins GJ, Haynes DR, Graves SE, et al. Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res. 2000;15(4):640–9.
Strauss SJ, Frezza AM, Abecassis N, et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(12):1520–36.
Casimiro S, Vilhais G, Gomes I, Costa L. The roadmap of RANKL/RANK pathway in cancer. Cells. 2021;10(8):1978. https://doi.org/10.3390/cells10081978.
National Comprehensive Cancer Network. Bone Cancer: NCCN clinical practice guidelines (Version 1.2024). 2023. https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed 7 Nov 2023.
Shanghai Jinmante Biotechnology Co. Ltd. Narlumosbart: Chinese prescribing information. Shanghai: Shanghai Jinmante Biotechnology Co. Ltd; 2023.
CSPC Pharmaceutical Group. Biologic License Application for narlumosbart for injection (JMT103) approved by the NMPA [media release]. 6 Sep 2023. https://doc.irasia.com/listco/hk/cspc/announcement/a230906a.pdf.
National Medical Products Administration. Narlumosbart Injection: NMPA listing information. 2023. https://www.nmpa.gov.cn/datasearch/search-info.html?nmpa=aWQ9ZTQ1OTllNGE5MDg1MTBhMDk4OTQ1NTdhYzc1MzhkMDMmaXRlbUlkPWZmODA4MDgxODNjYWQ3NTAwMTg0MDg4MWY4NDgxNzlm. Accessed 27 Oct 2023.
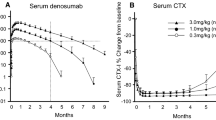
Liang X, Xue J, Ge X, et al. Safety, tolerability, and pharmacokinetics/pharmacodynamics of JMT103 in patients with bone metastases from solid tumors. Front Oncol. 2022;12: 971594.
Niu X, Wei F, Tu C, et al. Efficacy and safety of JMT103 in patients with giant cell tumor of bone: a multicenter, single-arm, open-label, phase Ib/II study [abstract no. 11526]. J Clin Oncol. 2021;39(15 Suppl):11526.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of Interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sohita Dhillon is a contracted employee of Adis International Ltd/Springer Nature and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhillon, S. Narlumosbart: First Approval. Drugs 84, 105–109 (2024). https://doi.org/10.1007/s40265-023-01985-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01985-3