Abstract
Rozanolixizumab (rozanolixizumab-noli; RYSTIGGO®) is a high affinity humanized immunoglobulin G4 monoclonal antibody directed against human neonatal Fc receptor (FcRn). Administered subcutaneously, it is being developed by UCB Pharma for the treatment of autoimmune diseases and received its first approval on 27 June 2023 in the USA for the treatment of generalized myasthenia gravis (gMG) in adults who are anti-acetylcholine receptor (AChR) or anti-muscle-specific kinase (MuSK) antibody positive. Rozanolixizumab is the first agent to be approved in the USA for both anti-AChR and anti-MuSK antibody-positive gMG. A regulatory assessment of rozanolixizumab for the treatment of gMG is currently underway in the EU and Japan. Clinical development is ongoing for the treatment of leucine-rich glioma-inactivated 1 autoimmune encephalitis, myelin oligodendrocyte glycoprotein (MOG) antibody disease and severe fibromyalgia syndrome. This article summarizes the milestones in the development of rozanolixizumab leading to this first approval for the treatment of gMG in adults who are anti-AChR or anti-MuSK antibody positive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.23929866. |
An FcRn antagonist being developed by UCB Pharma for the treatment of autoimmune diseases, including gMG |
Received its first approval on 27 June 2023 in the USA |
Approved for use in adults with gMG who are anti-AChR or anti-MuSK antibody positive |
1 Introduction
Myasthenia gravis (MG) is a chronic autoimmune disorder characterized by skeletal muscle weakness that worsens following periods of activity and improves after periods of rest [1]. Its presentation can be broadly classified as ocular or generalized, and it results from impaired transmission at the neuromuscular junction [1, 2]. Specifically, immunoglobulin G (IgG) antibodies [most commonly (up to 85% of generalized MG cases) to the post-synaptic acetylcholine receptor (AChR) itself and less often to other proteins (e.g. muscle-specific kinase; MuSK)] block the binding of acetylcholine to its receptor (thereby inhibiting acetylcholine-dependent signalling) and induce the internalization and destruction of AChR via complement activation mediated by the fragment crystallizable (Fc) domain of AChR antibodies [1,2,3].
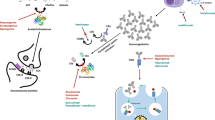
Currently there is no known cure for MG: its symptoms are controlled via long-term immunosuppression [1, 2]. Advances in drug development, immunopathology and translational medicine have resulted in the advent of novel biological agents that target antibody-mediated disease mechanisms [2, 3]. Such agents, among them neonatal Fc receptor (FcRn) inhibitors, show promise in overcoming the adverse event (AE) limitations associated with non-specific immunotherapies for the treatment of generalized MG (gMG) [2, 3]. FcRn plays a vital role in prolonging the half-life of IgG; blocking it prevents IgG recycling, thereby reducing the half-life and consequently the circulating levels of IgG [2].

Key milestones in the development of rozanolixizumab for the treatment of generalized myasthenia gravis. BLA Biologic License Application
Rozanolixizumab (rozanolixizumab-noli; RYSTIGGO®) is a humanized IgG4 monoclonal antibody directed against human FcRn [4]. Developed by UCB Pharma for the treatment of autoimmune diseases, rozanolixizumab received its first approval on 27 June 2023 for the treatment of gMG in adults who are anti-AChR or anti-MuSK antibody positive in the USA [4,5,6,7]. It is the first agent to be approved in the USA for both anti-AChR and anti-MuSK antibody-positive gMG [4]. In patients weighing < 50 kg, 50 kg to < 100 kg and ≥ 100 kg, respectively, the recommended dosage of rozanolixizumab is 420 mg, 560 mg and 840 mg (i.e. an infusion volume of 3 mL, 4 mL and 6 mL), administered as a subcutaneous infusion (at a pump rate of up to 20 mL/h) once weekly for 6 weeks [5]. Subsequent cycles should be administered based on clinical evaluation; the safety of initiating rozanolixizumab < 63 days after the start of the previous treatment cycle has not yet been established. A missed dose of rozanolixizumab may be administered up to 4 days after the scheduled dose, following which the original dosing schedule should be resumed. Rozanolixizumab may increase the risk of infection and thus should not be administered to patients with an active infection. It is also associated with transient reductions in IgG levels; thus, immunization with live-attenuated or live vaccines is not recommended during treatment and the need to administer age-appropriate immunizations as per immunization guidelines should be evaluated before initiating a new treatment cycle [5].
A regulatory assessment of rozanolixizumab for the treatment of gMG is currently underway in the EU and Japan; prior to this, the agent was granted orphan drug designation in these countries/regions [4]. Clinical development is ongoing for the treatment of leucine-rich glioma-inactivated 1 autoimmune encephalitis, myelin oligodendrocyte glycoprotein (MOG) antibody disease and severe fibromyalgia syndrome [8]. The chronic inflammatory demyelinating polyneuropathy and immune thrombocytopenia indications are not present in the UCB pipeline (as of July 2023) [8].
2 Scientific Summary
2.1 Pharmacodynamics
Rozanolixizumab is a humanized IgG4 monoclonal antibody [5]. It binds with high affinity (dissociation constant 23 and 34 pmol/L at pH 6.0 and pH 7.4; surface plasmon resonance analysis [9]) and specificity to human FcRn, thereby blocking the interaction of FcRn with IgG, which accelerates IgG catabolism and reduces circulating IgG levels [4, 5, 9].
In vitro, rozanolixizumab dose-dependently inhibited human FcRn-mediated recycling of human IgG (half-maximal inhibitory concentration 0.41 nmol/L) and did not induce cytokine release [9]. In human FcRn transgenic mice, and in cynomolgus monkeys, a single dose of rozanolixizumab was associated with a rapid decrease in plasma IgG levels; there was no reduction in plasma albumin levels (FcRn is responsible for salvaging and recycling albumin in endothelial and hematopoietic cells) outside the normal range [9].
In healthy volunteers participating in a first-in-human, phase I dose-escalating study (NCT02220153), single ascending doses of intravenous or subcutaneous rozanolixizumab were associated with sustained dose-dependent reductions in serum IgG levels of up to 50% [10]. Maximal reductions were achieved by day 7–10 after which IgG levels increased, returning to baseline levels by day 57 [10]. In adults with moderate to severe gMG participating in a two-period, multinational phase IIa study (NCT03052751), varying dosage regimens (see Sect. 2.3.2) of subcutaneous rozanolixizumab were associated with reductions in serum IgG and AChR antibody levels of 59–68% [11]. In adults with AChR or MuSK autoantibody-positive gMG participating in a multinational phase III study [MycarinG (NCT03971422)], the median maximum reduction from baseline in total IgG levels was 73% with rozanolixizumab 7 mg/kg and 79% with rozanolixizumab 10 mg/kg (both rozanolixizumab dosages were administered subcutaneously once weekly for 6 weeks) versus 9% with placebo [12]. Moreover, the median maximum reduction from baseline in AChR autoantibody levels was 73% and 82% versus 25% in the respective groups. Reductions in total IgG and AChR levels were seen as early as day 8 and 15, respectively, in both rozanolixizumab groups; the levels continued to decline throughout the remainder of the treatment period, following which they gradually increased and were approaching baseline levels by day 99 [12].
2.2 Pharmacokinetics
The pharmacokinetics of rozanolixizumab are nonlinear [10]. Following the subcutaneous administration of rozanolixizumab, increases in exposure were greater than dose-proportional over a 1–20 mg/kg dose range and, in healthy volunteers, maximum plasma concentrations were reached after ≈ 2 days [5]. Rozanolixizumab is expected to be degraded by proteolytic enzymes into small peptides and amino acids [5].
According to a population pharmacokinetic (PPK) analysis, the pharmacokinetics of rozanolixizumab were not affected by age, sex or race [5]. While dedicated pharmacokinetic studies have not been conducted in patients with hepatic or renal impairment, neither is expected to affect the pharmacokinetics of rozanolixizumab. Indeed, according to a PPK analysis that included participants with mild to moderate renal impairment, renal function (estimated glomerular filtration rate 38–161 mL/min/1.73 m2) had no clinically relevant effect on the apparent clearance of rozanolixizumab [5].
While clinical drug interaction studies have not been performed with rozanolixizumab, interactions with concomitant medications that are substrates, inducers or inhibitors of cytochrome P450 (CYP) enzymes are unlikely (as rozanolixizumab is not metabolized by CYP enzymes) [5]. However, rozanolixizumab may decrease the systemic exposure and thus effectiveness of medications (e.g. immunoglobulin products, monoclonal antibodies, or antibody derivatives containing the human Fc domain of the IgG subclass) that bind to human FcRn. Patients receiving such medications should therefore be closely monitored for reduced effectiveness. If long-term concomitant use of such medications is essential, consider discontinuing rozanolixizumab and using alternative treatments [5].
2.3 Therapeutic Trials
2.3.1 Phase III Study
Therapy with subcutaneous rozanolixizumab 7 mg/kg and 10 mg/kg (administered once weekly for 6 weeks) was associated with statistically significant and clinically meaningful improvements from baseline in disease-related endpoints in adults with AChR or MuSK autoantibody-positive gMG participating in a randomized, double-blind, placebo-controlled, multinational phase III study [MycarinG (NCT03971422)] [12].
Features and properties of rozanolixizumab
Alternative names | Rozanolixizumab-noli; RYSTIGGO; UCB-7665 |
Class | Anti-inflammatories; Monoclonal antibodies |
Mechanism of action | Neonatal Fc receptor antagonist |
Route of administration | Intravenous; subcutaneous |
Pharmacodynamics | Humanized immunoglobulin (Ig) G4 monoclonal antibody that binds to the human neonatal Fc receptor and blocks its interaction with IgG, thereby accelerating IgG catabolism and thus reducing circulating IgG levels |
Pharmacokinetics | Nonlinear pharmacokinetics; exposure increases in a greater than dose-proportional manner over a 1–20 mg/kg dose range and maximum plasma concentrations were reached after ≈ 2 days following subcutaneous administration |
Most frequent adverse events | Headache, diarrhoea, pyrexia and nausea |
ATC codes | |
WHO ATC code | L04A-G16 (Rozanolixizumab) |
EphMRA ATC code | B6 (All Other Haematological Agents); M1 (Anti-Inflammatory and Anti-Rheumatic Products); N7X (All other CNS drugs) |
The least-squares mean (LSM) change from baseline to day 43 in the Myasthenia Gravis Activities of Daily Living (MG-ADL) score (primary endpoint) was significantly greater with both rozanolixizumab 7 mg/kg and 10 mg/kg (n = 66 and 67) [− 3.37 and − 3.40] than with placebo (n = 67) [− 0.78; LSM between-group differences − 2.59 (95% CI − 4.09 to − 1.25) and − 2.62 (95% CI − 3.99 to − 1.16); both p < 0.0001] [12]. A LSM between-group difference of > 1.5 was considered to be clinically relevant. Sensitivity analyses demonstrated that the results of the primary analysis were robust. Improvements from baseline in MG-ADL scores were seen as early as day 8. Findings for this endpoint were consistent regardless of sex or autoantibody status [e.g. AChR autoantibody-positive: LSM between-group difference from placebo: − 1.94 (97.5% CI − 3.06 to − 0.81) for rozanolixizumab 7 mg/kg and − 2.26 (97.5% CI − 3.39 to − 1.13) for rozanolixizumab 10 mg/kg; MuSK autoantibody-positive: LSM between-group difference from placebo: − 9.56 (97.5% CI − 15.25 to − 3.87) and − 6.45 (− 11.03 to − 1.86)] in prespecified subgroup analyses [12].
In terms of secondary endpoints, statistically significant LSM between-group differences favouring both rozanolixizumab 7 mg/kg and 10 mg/kg over placebo were seen for the LSM change from baseline to day 43 in the MG-Composite (MG-C) scale score [− 5.93 and − 7.55 vs − 2.03; − 3.90 (95% CI − 6.63 to − 1.25) and − 5.53 (95% CI − 8.30 to − 2.97); p = 0.0004 and p < 0.0001]; the quantitative myasthenia gravis (QMG) scale score [− 5.40 and − 6.67 vs − 1.92; − 3.48 (95% CI − 5.61 to − 1.58) and − 4.76 (95% CI − 6.82 to − 2.86); p < 0.0001 and < 0.0001] [12]. Statistically significant LSM between-group differences favouring both rozanolixizumab 7 mg/kg and 10 mg/kg over placebo were also seen for the following secondary endpoints: the Myasthenia Gravis Symptoms Patient-Reported Outcome (PRO) scale scores of muscle weakness fatigability [− 23.03 and − 25.75 vs − 10.59; − 12.44 (95% CI − 21.80 to − 4.09) and − 15.16 (95% CI − 23.60 to − 6.45); p = 0.0003 and p < 0.0001], physical fatigue [− 19.29 and − 25.46 vs − 10.64; − 8·65 (95% CI − 18.06 to − 0.13) and − 14.82 (95% CI − 23.76 to − 5.94); p = 0.0120 and 0.0002] and bulbar muscle weakness [− 14.84 and − 14.22 vs − 3.52; − 11.32 (95% CI − 18.96 to − 5.00) and − 10.71 (95% CI − 17.79 to − 4.00); p < 0.0001 and < 0.0001]. Improvements from baseline in MG-C scale, QMG scale and Myasthenia Gravis Symptoms PRO scale scores were seen as early as day 8 [12].
Clinically meaningful improvements of ≥ 2 points for MG-ADL (71.9% and 69.4% vs 31.3%; secondary endpoint) and ≥ 3 points for MG-C (60.9% and 74.2% vs 40.6%) and QMG (54.7% and 72.6% vs 39.1%) were achieved by approximately half to three-quarters of rozanolixizumab 7 mg/kg and 10 mg/kg recipients and one-third to two-fifths of placebo recipients at day 43 [12]. Moreover, minimal symptom expression (defined as an MG-ADL score of 0 or 1) was achieved by 26% of patients in the rozanolixizumab 7 mg/kg group, 28% of patients in the rozanolixizumab 10 mg/kg group and 3% of patients in the placebo group. Rescue therapy (intravenous immunoglobulin or plasma exchange for disease worsening; administered at the investigator’s discretion) was not required by any patient in the two rozanolixizumab groups during the treatment period [12].
MycarinG consisted of a 6-week treatment period followed by an 8-week observation period [12]. Patients eligible for this study had a MG-ADL score of ≥ 3 (with ≥ 3 points for non-ocular symptoms), a QMG scale score of ≥ 11, a bodyweight of ≥ 35 kg and had been considered for treatment with additional therapy such as intravenous immunoglobulin or plasma exchange. MG-ADL scores range from 0 to 24, QMG scale scores range from 0 to 39 and MG-C scale scores range from 0 to 50, with higher scores for each indicating more severe disability. Patients with severe oropharyngeal or respiratory weakness, a clinically relevant active infection or recent serious infection, or a total IgG concentration of ≤ 5.5 g/L were among those excluded from MycarinG. Concomitant therapies [cholinesterase inhibitors (stable dose not required), oral corticosteroids (stable for 4 weeks before baseline), azathioprine, ciclosporin, methotrexate, mycophenolate mofetil, and tacrolimus] were permitted if used for the previous 6 months and with a stable dose for 2 months prior to baseline. Randomization was stratified by the presence of AChR or MuSK autoantibodies. At baseline, 90% and 11% of 200 patients were AChR or MuSK autoantibody positive, the mean MG-ADL score was 8.3 and the mean QMG scale score was 15.6. An open-label extension study of MycarinG has been completed (NCT04124965) and another is underway (NCT04650854) [12]. Patients who completed the observation period of MycarinG or whose disease severity worsened (according to the investigator) during it could enrol in one or both open-label extensions: NCT04124965 then NCT04650854, or NCT04650854 [12, 13]. Those who received rescue therapy during the observation period discontinued MycarinG and were not eligible for the OLE studies [12].
Repeated cyclic administration of subcutaneous rozanolixizumab resulted in sustained benefits across multiple MG-specific outcomes according to a pooled analysis of data from patients with ≥ 2 symptom-driven cycles (up to 6 six-week cycles) who participated in MycarinG, the first 6 weeks of NCT04124965, or NCT04650854 (interim analysis) [13]. In cycles 1, 2, 3, 4, 5 and 6, respectively, the mean change from baseline to day 43 was − 3.7, − 3.9, − 3.4, − 3.8, − 3.9 and − 4.5 points in the MG-ADL score (n = 127, 127, 98, 75, 51 and 32) and − 5.4, − 4.7, − 4.7, − 5.1, − 4.5 and − 6.3 in the QMG scale score (n = 127, 125, 97, 74, 51 and 32). Reductions in the MG-C scale score were also consistent across the cycles. In NCT04124965, patients received up to 52 weeks of weekly rozanolixizumab infusions. In NCT04650854, patients received an initial rozanolixizumab cycle, with subsequent cycles administered on symptom worsening (e.g. MG-ADL score increase of ≥ 2 points, QMG scale score increase of ≥ 3 points) at the investigator’s discretion. Of the 127 patients, 69 received rozanolixizumab 7 mg/kg and 58 received rozanolixizumab 10 mg/kg. Patients who participated for > 1 year initiated a median of four cycles per year in the first year [13].
2.3.2 Phase II Study
Therapy with subcutaneous rozanolixizumab 7 mg/kg (administered once weekly for 3 weeks; n = 21) did not significantly improve the QMG scale score from baseline to day 29 (primary endpoint) compared with placebo (n = 22) in adults with moderate to severe gMG participating in a two-period (period 1: days 1–29; period 2: days 29–43), randomized, double-blind, placebo-controlled, multinational phase IIa study (NCT03052751) [11]. At day 29, the LSM change from baseline was − 1.8 in the rozanolixizumab group and − 1.2 in the placebo group [LSM between-group difference − 0.7 (95% upper CI 0.8)]. In terms of secondary endpoints, the LSM change from baseline to day 29 in the rozanolixizumab and placebo groups was − 1.8 and − 0.4 [LSM between-group difference − 1.4 (95% upper CI − 0.4)] in the MG-ADL score and − 3.1 and − 1.2 (LSM between-group difference − 1.8 (95% upper CL 0.4)] in the MG-C scale score [11].
Efficacy outcomes continued to improve in period 2 of the study [11]. The continuation of rozanolixizumab 7 mg/kg in 10 patients was associated with further improvements in QMC scale, MG-ADL and MG-C scale scores: the respective scores reached nadir 21, 21 and 14 days after the re-initiation of rozanolixizumab therapy and returned towards baseline values during the observation period. In the 10 patients re-randomized from rozanolixizumab 7 mg/kg to 4 mg/kg, nadir was achieved for all three measures 21 days following administration of the first 4 mg/kg dose. Improvements in QMC scale, MG-ADL and MG-C scale scores were seen in the placebo recipients re-randomized to rozanolixizumab 7 mg/kg or 4 mg/kg [11].
NCT03052751 consisted of two treatment periods: during period 1 (days 1–29), patients received rozanolixizumab 7 mg/kg once weekly for 3 weeks; during period 2 (days 29–43), patients were re-randomized to either rozanolixizumab 7 mg/kg or 4 mg/kg once weekly for 3 weeks. Following the completion of period 2, patients were observed on days 44–99 [11]. Patients eligible for this study had a documented diagnosis of gMG with evidence of elevated anti-AChR or anti- MuSK autoantibodies prior to screening, a QMG scale score of ≥ 11 at baseline, a serum total IgG level of > 6 g/L at screening and had been considered for treatment with intravenous immunoglobulin or plasma exchange. Patients with MG that only affected the ocular muscles were among those excluded [11].
2.4 Adverse Events
Subcutaneous rozanolixizumab was generally well tolerated in phase III [12] and II [11] studies.
In the phase III MycarinG study [12], treatment-emergent AEs (TEAEs) were reported in 81% of 64 rozanolixizumab 7 mg/kg recipients, 83% of 69 rozanolixizumab 10 mg/kg recipients and 67% of 67 placebo recipients. Most TEAEs were mild to moderate; severe TEAEs occurred in 5%, 19% and 4% of patients in the respective groups. The most frequently reported TEAEs were headache (45%, 38% and 19% in the rozanolixizumab 7 mg/kg, rozanolixizumab 10 mg/kg and placebo groups, respectively), diarrhoea (25%, 16% and 13%), pyrexia (13%, 20% and 1%) and nausea (8%, 12% and 7%) [12]. Rozanolixizumab may increase the risk of infection [5]. In MycarinG, infections occurred in 16%, 30% and 19% of patients in the rozanolixizumab 7 mg/kg, rozanolixizumab 10 mg/kg and placebo groups; none of the patients in either rozanolixizumab group experienced a severe or serious infection, while one patient in the placebo group developed a serious infection (COVID-19 pneumonia) [12]. Treatment-related (as assessed by the investigator) TEAEs were reported in 50%, 57% and 33% of patients in the rozanolixizumab 7 mg/kg, rozanolixizumab 10 mg/kg and placebo groups, respectively. Serious TEAEs were reported in 8% of rozanolixizumab 7 mg/kg recipients, 10% of rozanolixizumab 10 mg/kg recipients and 9% of placebo recipients but no serious anaphylactic, hypersensitivity or injection-site reactions occurred. Treatment discontinuation due to TEAEs occurred in two (3%) patients receiving rozanolixizumab 7 mg/kg (arthralgia and headache), four (6%) patients receiving rozanolixizumab 10 mg/kg (deep vein thrombosis, diarrhoea, metastatic squamous cell carcinoma, oral herpes, pruritus, upper abdominal pain and vomiting; some patients reported > 1 event) and two (3%) patients receiving placebo (myasthenia gravis and myasthenia gravis crisis). No patients died in MycarinG [12].
Key clinical trials of rozanolixizumab
Drug(s) | Indication | Phase | Status | Location(s) | Identifier | Sponsor |
|---|---|---|---|---|---|---|
Rozanolixizumab, placebo | Generalized myasthenia gravis | III | Completed | Multinational | NCT03971422 (MycarinG) | UCB Biopharma SRL |
Rozanolixizumab | Generalized myasthenia gravis | III | Completed | Multinational | NCT04124965 | UCB Biopharma SRL |
Rozanolixizumab | Primary immune thrombocytopenia | III | Completed | Multinational | NCT04596995 (myOpportunITy3) | UCB Biopharma SRL |
Rozanolixizumab | Generalized myasthenia gravis | III | Active, not recruiting | Multinational | NCT04650854 | UCB Biopharma SRL |
Rozanolixizumab | Generalized myasthenia gravis | III | Recruiting | Multinational | NCT05681715 | UCB Biopharma SRL |
Rozanolixizumab, placebo | Myelin oligodendrocyte glycoprotein antibody-associated disease | III | Recruiting | Multinational | NCT05063162 (cosMOG) | UCB Biopharma SRL |
Rozanolixizumab | Primary immune thrombocytopenia | II | Completed | Multinational | NCT02718716 | UCB Biopharma SPRL |
Rozanolixizumab, placebo | Generalized myasthenia gravis | II | Completed | Multinational | NCT03052751 | UCB Biopharma SPRL |
Rozanolixizumab | Chronic inflammatory demyelinating polyradiculoneuropathy | II | Completed | Multinational | NCT03861481 (MyCIDPchoice) | UCB Biopharma SPRL |
Rozanolixizumab | Chronic inflammatory demyelinating polyradiculoneuropathy | II | Completed | Multinational | NCT04051944 | UCB Biopharma SRL |
Rozanolixizumab | Leucine-rich glioma-inactivated 1 autoimmune encephalitis | II | Recruiting | Multinational | NCT04875975 | UCB Biopharma SRL |
Rozanolixizumab, placebo | Severe fibromyalgia syndrome | II | Recruiting | UK | NCT05643794 | UCB Biopharma SRL |
Rozanolixizumab | Chronic inflammatory demyelinating polyradiculoneuropathy | Expanded access | Recruiting | Multinational | NCT05014724 | UCB Biopharma SRL |
In a pooled analysis of data from patients with ≥ 2 symptom-driven cycles (up to 6 six-week cycles) who participated in MycarinG, the first 6 weeks of NCT04124965, or NCT04650854 (interim analysis), 89.9% of 188 patients receiving ≥ 1 cycle of rozanolixizumab experienced ≥ 1 TEAE, with most TEAEs being mild to moderate in severity [13]. In MycarinG and its extension studies, 48% of 196 patients who received treatment with rozanolixizumab reported infections [5]. The most frequently reported (frequency ≥ 5%) infections were upper respiratory tract infections (17% of patients), COVID-19 infection (14%), urinary tract infections (9%) and herpes simplex (6%). Serious infections were reported in 4% of patients, treatment discontinuation due to infection in 3% of patients and death due to infection in 1.5% of patients [5].
Anti-drug antibodies (ADAs) and neutralizing antibodies were detected in 37% and 21% of 133 patients at the end of the observation period (i.e. after one 6-week treatment cycle) in MycarinG [5]. While an up to 60% reduction in trough rozanolixizumab concentrations was seen in ADA-positive patients compared with ADA-negative patients, ADAs appear to have no clinically meaningful impact on the efficacy of rozanolixizumab [5].
2.5 Ongoing Clinical Trials
In patients with gMG, the multinational, phase III extension study NCT04650854 is currently ongoing, while recruitment is underway for another multinational, phase III crossover study (NCT05681715), which is evaluating the self-administration of rozanolixizumab via two different methods. A multinational phase III study in adults with myelin oligodendrocyte glycoprotein antibody-associated disease [cosMOG (NCT05063162)] and two multinational phase II studies in adults with leucine-rich glioma inactivated 1 autoimmune encephalitis (NCT04875975) and severe fibromyalgia syndrome (NCT05643794) are likewise recruiting.
Change history
20 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40265-023-01960-y
References
National Institute of Neurological Disorders and Stroke. Myasthenia Gravis Fact Sheet. 2023. https://www.ninds.nih.gov/myasthenia-gravis-fact-sheet. Accessed 19 Jul 2023.
Menon D, Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs. 2022;82(8):865–87.
Lunemann JD. Getting specific: targeting Fc receptors in myasthenia gravis. Nat Rev Neurol. 2021;17(10):597–8.
UCB Inc. UCB announces U.S. FDA approval of RYSTIGGO® (rozanolixizumab-noli) for the treatment of adults with generalized myasthenia gravis [media release]. 27 Jun 2023. https://www.ucb.com/stories-media/Press-Releases
UCB Inc. RYSTIGGO® (rozanolixizumab-noli) injection, for subcutaneous use: US prescribing information. 2023. https://www.ucb-usa.com/RYSTIGGO-prescribing-information.pdf. Accessed 18 Jul 2023.
US FDA. FDA Roundup: June 27, 2023 [media release]. 27 Jun 2023. https://www.fda.gov/news-events/press-announcements/fda-roundup-june-27-2023.
UCB Inc. UCB presents final results from phase II study of rozanolixizumab in primary immune thrombocytopenia (ITP) at 2019 ASH Annual Meeting [media release]. 9 Dec 2019. http://www.ucb.com.
UCB Inc. UCB pipeline. 2023. https://www.ucb.com/our-science/pipeline. Accessed 26 Jul 2023.
Smith B, Kiessling A, Lledo-Garcia R, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. 2018;10(7):1111–30.
Kiessling P, Lledo-Garcia R, Watanabe S, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci Transl Med. 2017;9(414):1–12.
Bril V, Benatar M, Andersen H, et al. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis: a phase 2 randomized control trial. Neurology. 2021;96(6):e853–65.
Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383–94.
Bril V, Druzdz A, Grosskreutz J, et al. Long-term efficacy and safety of symptom-driven cyclic rozanolixizumab treatment in patients with generalized myasthenia gravis: a pooled analysis of a phase 3 study and two open-label extension studies [abstract no. P1-5.012]. Neurology. 2023;100(17 Suppl 2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan M. Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to this article and are responsible for its content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original article has been revised due to retrospective open choice order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hoy, S.M. Rozanolixizumab: First Approval. Drugs 83, 1341–1347 (2023). https://doi.org/10.1007/s40265-023-01933-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01933-1