Abstract
Background
Posaconazole is widely used for the prophylaxis and treatment of invasive fungal diseases. Because of the limited and variable absorption of the initially available oral suspension, a delayed-release tablet and intravenous formulation were developed.
Objective
This study aimed to characterize the pharmacokinetics, including the absolute oral bioavailability, of all posaconazole formulations in healthy volunteers.
Methods
Data from 182 healthy volunteers with 3898 densely sampled posaconazole concentrations were pooled from eight phase I clinical studies on the three formulations of various single and multiple dosage regimens between 50 and 400 mg. Analysis and simulations were performed using NONMEM 7.5.0. In the covariate analysis, the influence of food (fed vs fasted), nonlinearity, and for the delayed-release tablet, comedication (antacid, ranitidine, esomeprazole, and metoclopramide) were tested.
Results
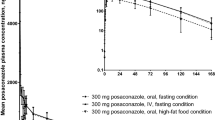
A two-compartment model with respectively, four and eight absorption transit compartments, best described the profiles of the oral suspension and delayed-release tablet. For the suspension, both a food effect and a dose-dependent nonlinear bioavailability were quantified, resulting in lower bioavailability when fasted or at a higher dose. The typical bioavailability of the suspension at 100 mg and 400 mg was derived to be respectively, 17.1% and 10.1% under fasted conditions and 59.1% and 49.2% under fed conditions. The absolute bioavailability of the delayed-release tablet was 58.8% (95% confidence interval 33.2–80.4) under fasted conditions and approached complete absorption under fed conditions for dosages up to 300 mg. Food intake reduced the absorption rate constant of the suspension by 52.2% (confidence interval 45.2–59.2). The impact of comedication on the absorption of the delayed-release tablet was not statistically significant. Model-based simulations indicate that under fed conditions, the licensed dosages of the three formulations yield a steady-state trough concentration ≥ 0.7 mg/L in over 90% of healthy volunteers. About 35% of healthy volunteers who receive the licensed 300-mg delayed-release tablet under fasted conditions do not achieve this target, while for the suspension this percentage varies between 55 and 85%, depending on the dose.
Conclusions
For both oral posaconazole formulations, we quantified bioavailability and absorption rate, including food effects, in healthy volunteers. The pharmacokinetic superiority of the delayed-release tablet was demonstrated under both fed and fasted conditions, compared with the oral suspension. The impact of food on the bioavailability of the delayed-release tablet was larger than anticipated, suggesting that administering the delayed-release tablet with food enhances absorption.




Similar content being viewed by others
References
EMA. Summary of posaconazole characteristics. 2022 January 6, 2022. https://www.ema.europa.eu/en/documents/product-information/noxafil-epar-product-information_en.pdf. Accessed 1 Feb 2022.
European Committee on Antimicrobial Susceptibility Testing. Posaconazole: rationale for the EUCAST clinical breakpoints, version 3.0. 4 February, 2020. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Posaconazole_RD_v3.0_final_final_18_02.pdf. Accessed 1 Feb 2022.
FDA. Noxafil instruction. March 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205053s1lbl.pdf. Accessed 1 Feb 2022.
Chen L, Krekels EHJ, Verweij PE, Buil JB, Knibbe CAJ, Brüggemann RJM. Pharmacokinetics and pharmacodynamics of posaconazole. Drugs. 2020;80(7):671–95.
Ullmann AJ, Cornely OA, Burchardt A, Hachem R, Kontoyiannis DP, Topelt K, et al. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother. 2006;50(2):658–66.
Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol. 2004;57(2):218–22.
Krishna G, Moton A, Ma L, Medlock MM, McLeod J. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53(3):958–66.
Cornely OA, Duarte RF, Haider S, Chandrasekar P, Helfgott D, Jimenez JL, et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother. 2016;71(3):718–26.
Duarte RF, Lopez-Jimenez J, Cornely OA, Laverdiere M, Helfgott D, Haider S, et al. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother. 2014;58(10):5758–65.
Kraft WK, Chang PS, van Iersel ML, Waskin H, Krishna G, Kersemaekers WM. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother. 2014;58(7):4020–5.
Jang SH, Colangelo PM, Gobburu JV. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther. 2010;88(1):115–9.
Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–59.
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–47.
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl. 1):e1-38.
Krishna G, Ma L, Martinho M, O’Mara E. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole in new tablet and capsule formulations relative to oral suspension. Antimicrobi Agents Chemother. 2012;56(8):4196–201.
EMA. Posaconazole tablet assessment report: EPAR-scientific discussion-extension. 20 February, 2014. EMEA/H/C/000610/X/0028. https://www.ema.europa.eu/en/documents/variation-report/noxafil-h-c-610-x-0028-epar-scientific-discussion-extension_en.pdf. Accessed 1 Feb 2022.
EMA. Posaconazole injection assessment report: EPAR assessment report: variation. 24 Jul 2014. EMEA/H/C/000610/X/0033. https://www.ema.europa.eu/en/documents/variation-report/noxafil-h-c-610-x-0033-epar-assessment-report-variation_en.pdf. Accessed 1 Feb 2022.
Kersemaekers WM, van Iersel T, Nassander U, O’Mara E, Waskin H, Caceres M, et al. Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects. Antimicrob Agents Chemother. 2015;59(2):1246–51.
Bruggemann RJ, van Luin M, Colbers EP, van den Dungen MW, Pharo C, Schouwenberg BJ, et al. Effect of posaconazole on the pharmacokinetics of fosamprenavir and vice versa in healthy volunteers. J Antimicrob Chemother. 2010;65(10):2188–94.
Wasmann RE, Smit C, van Donselaar MH, van Dongen EPA, Wiezer RMJ, Verweij PE, et al. Implications for IV posaconazole dosing in the era of obesity. J Antimicrob Chemother. 2020;75(4):1006–13.
Courtney R, Pai S, Laughlin M, Lim J, Batra V. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother. 2003;47(9):2788–95.
Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol. 2013;2(6): e50.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35(4):401–21.
Rousseau A, Léger F, Le Meur Y, Saint-Marcoux F, Paintaud G, Buchler M, et al. Population pharmacokinetic modeling of oral cyclosporin using NONMEM: comparison of absorption pharmacokinetic models and design of a Bayesian estimator. Ther Drug Monit. 2004;26(1):23–30.
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26.
Ruiz-Garcia A, Tan W, Li J, Haughey M, Masters J, Hibma J, et al. Pharmacokinetic models to characterize the absorption phase and the influence of a proton pump inhibitor on the overall exposure of dacomitinib. Pharmaceutics. 2020;12(4):330.
Zhou H. Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol. 2003;43(3):211–27.
Hare RK, Gertsen JB, Astvad KMT, Degn KB, Løkke A, Stegger M, et al. In vivo selection of a unique tandem repeat mediated azole resistance mechanism (TR120) in Aspergillus fumigatus CYP51A. Denmark Emerg Infect Dis. 2019;25(3):577–80.
Hens B, Pathak SM. In silico modeling approach for the evaluation of gastrointestinal dissolution, supersaturation, and precipitation of posaconazole. Mol Pharm. 2017;14(12):4321–33.
Krishna G, Ma L, Vickery D, Yu X, Wu I, Power E, et al. Effect of varying amounts of a liquid nutritional supplement on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob Agents Chemother. 2009;53(11):4749–52.
Dieringer TD, Schaenman JM, Davis MR. Enteral feeding tube administration with therapeutic drug monitoring of crushed posaconazole tablets and opened isavuconazonium sulfate capsules. J Antimicrob Chemother. 2022;77(5):1417–23.
EMA. Noxafil EPAR assessment report. 16 September, 2021. https://www.ema.europa.eu/en/documents/variation-report/noxafil-h-c-610-ii-0062-epar-assessment-report-variation_en.pdf. Accessed 25 May 2022.
Kohl V, Muller C, Cornely OA, Abduljalil K, Fuhr U, Vehreschild JJ, et al. Factors influencing pharmacokinetics of prophylactic posaconazole in patients undergoing allogeneic stem cell transplantation. Antimicrob Agents Chemother. 2010;54(1):207–12.
Petitcollin A, Boglione-Kerrien C, Tron C, Picard S, Lalanne S, Nimubona S, et al. Population pharmacokinetics and monte-carlo simulations of posaconazole administered as tablets in a real-life cohort of patients with hematological malignancies: towards dose reduction? Fundam Clin Pharmacol. 2017;31:19.
Vehreschild JJ, Muller C, Farowski F, Vehreschild MJ, Cornely OA, Fuhr U, et al. Factors influencing the pharmacokinetics of prophylactic posaconazole oral suspension in patients with acute myeloid leukemia or myelodysplastic syndrome. Eur J Clin Pharmacol. 2012;68(6):987–95.
Dolton MJ, Bruggemann RJ, Burger DM, McLachlan AJ. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis. Antimicrob Agents Chemother. 2014;58(11):6879–85.
Pena-Lorenzo D, Rebollo N, Sanchez-Hernandez JG, Zarzuelo-Castaneda A, Vazquez-Lopez L, Otero MJ, et al. Population pharmacokinetics of a posaconazole tablet formulation in transplant adult allogeneic stem cell recipients. Eur J Pharm Sci. 2022;1(168): 106049.
van Iersel M, Rossenu S, de Greef R, Waskin H. A population pharmacokinetic model for a solid oral tablet formulation of posaconazole. Antimicrob Agents Chemother. 2018;62(7):e02465-e2517.
AbuTarif MA, Krishna G, Statkevich P. Population pharmacokinetics of posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Curr Med Res Opin. 2010;26(2):397–405.
Storzinger D, Borghorst S, Hofer S, Busch CJ, Lichtenstern C, Hempel G, et al. Plasma concentrations of posaconazole administered via nasogastric tube in patients in a surgical intensive care unit. Antimicrob Agents Chemother. 2012;56(8):4468–70.
Jansen AME, Muilwijk EW, Van Der Velden WJFM, Maertens JA, Aerts R, Colbers A, et al. Posaconazole bioavailability of the solid oral tablet is reduced during severe intestinal mucositis. Clin Microbiol Infect. 2022;28(7):1003–9.
Gubbins PO, Krishna G, Sansone-Parsons A, Penzak SR, Dong L, Martinho M, et al. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob Agents Chemother. 2006;50(6):1993–9.
Howard SJ, Lestner JM, Sharp A, Gregson L, Goodwin J, Slater J, et al. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J Infect Dis. 2011;203(9):1324–32.
Lepak AJ, Marchillo K, Vanhecker J, Andes DR. Posaconazole pharmacodynamic target determination against wild-type and Cyp51 mutant isolates of Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2013;57(1):579–85.
Mavridou E, Bruggemann RJ, Melchers WJ, Mouton JW, Verweij PE. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob Agents Chemother. 2010;54(2):860–5.
Kuiken NSS, Rings EHHM, Blijlevens NMA, Tissing WJE. Biomarkers and non-invasive tests for gastrointestinal mucositis. Support Care Cancer. 2017;25(9):2933–41.
EMA. Noxafil: EPAR - product information. 22 February, 2022. EMEA/H/C/000610 - II/0067. https://www.ema.europa.eu/en/documents/product-information/noxafil-epar-product-information_en.pdf. Accessed 19 May 2022.
Acknowledgments
Data were shared under a contract between Merck & Co and Radboud University Medical Center. We thank Merck & Co., Inc., Kenilworth, NJ, USA for sharing their data on healthy volunteers. We thank all the healthy volunteers involved in these trials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work of Lu Chen was supported by the China Scholarship Council.
Conflicts of Interest/Competing Interests
No disclosures are applicable for this work. Disclosures outside of this work: RJB has served as a consultant to Astellas Pharma, Inc., F2G, Amplyx, Gilead Sciences, Merck Sharp & Dohme Corp., Mundipharma, and Pfizer, Inc., and has received unrestricted and research grants from Astellas Pharma, Inc., Gilead Sciences, Merck Sharp & Dohme Corp., and Pfizer, Inc. All contracts were through Radboud University Medical Center, and all payments were invoiced by Radboud University Medical Center. None of the other authors has a conflict of interest to declare.
Ethics Approval
Each clinical study involved in this paper received ethics approval.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Part of the data that support the findings of this study is available from Radboud University Medical Center but restrictions apply to the availability of these data. Data from Merck & Co were obtained under confidentiality and were used under license for the current study, thus are not publicly available.
Code Availability
The NONMEM code for the final model can be found in the ESM.
Authors’ Contributions
Conception and design of the research: LC, EHJK, CAJK, and RJB; data collection: RJB; data analysis: LC and ARH; interpretation of findings: LC, EHJK, ARH, CAJK, and RJB; drafting the manuscript: LC; critical revision of manuscript: EHJK, ARH, CAJK, and RJB; and approval of the final manuscript: LC, EHJK, ARH, CAJK, and RJB.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Krekels, E.H.J., Heijnen, A.R. et al. An Integrated Population Pharmacokinetic Analysis for Posaconazole Oral Suspension, Delayed-Release Tablet, and Intravenous Infusion in Healthy Volunteers. Drugs 83, 75–86 (2023). https://doi.org/10.1007/s40265-022-01819-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01819-8