Abstract
Introduction
There have been substantial changes in the nature of reporting pathways and review of suspected adverse drug reactions (ADRs) in Australia since the establishment of the now defunct Advisory Committee on Safety of Medicines early in 2010.
Objectives
The aim of this study was to (1) examine the reporting in Australia of suspected ADRs from various sources, including general practitioners (GPs), since 1990; (2) compare the reporting of Australian GPs with that in two other countries (New Zealand and the United Kingdom [UK]) with comparable safety monitoring programmes for the period 2007–2019; and (3) explore the extent to which Australian reporting of suspected adverse reactions has motivated communication to healthcare professionals in the period 1995–2019.
Methods
Annual reporting of sources of ADRs in Australia were obtained from Government reports, the Australian Statistics in Medicines and Therapeutic Goods Administration (TGA) websites. Details of the annual reporting by GPs in the UK were obtained from published sources and have been provided on request by the Medicines and Healthcare products Regulatory Agency. Details of the annual reporting by GPs in New Zealand were provided on request from the Centre for Adverse Reaction Monitoring. All issues of the Australian Adverse Drug Reactions Bulletin were accessed from the National Library of Australia, and issues of the Medicines Safety Update from February 1995 to December 2019 were accessed online from the TGA website. Each issue was searched to identify and score safety advisories.
Results
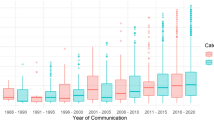
From 1990 to 2002 in Australia, overall reporting gradually increased, and the three major groups of reporters (GPs, hospitals and sponsors) each contributed about 30%. The relative contributions to reporting changed in the period 2002 to 2009. There was then a steep fall in reporting from GPs and the start of a very marked increase in reporting from product sponsors. GP reporting in Australia was lower than the two other comparable countries (New Zealand and the UK), and continues to fall, while in the UK at least, GP reporting is rising. The analysis of safety advisories shows a relatively stable Australian content from 1995 to 2008, followed by a sharp decline, so that by 2019 and 2020 there was barely any Australian reporting-driven content. In 1995 and 1996, Australian reports of suspected adverse reactions were the sole apparent reason for the publication of safety advisories. From 1997 to about 2008, Australian reports of suspected adverse reactions were the major reason for publication, but after this time, Australian reports became less important. During this later period, the apparent motive for publication of the safety advisory shifted to being based primarily on a publication in the medical literature, or publicity, but was sometimes based on an overseas regulator’s advice or action, or action by a product sponsor.
Conclusion
It is our contention that the decline in GP reporting in Australia and the current paucity in details of Australian reports in safety advisories are closely linked.




Similar content being viewed by others
References
Department of Health, Housing, Local Government and Community Services. Annual Report 1992-1993 Table 22; p535. https://nla.gov.au:443/tarkine/nla.obj-1681856593. Accessed 7 Nov 2021.
The Pharmaceutical Benefits Scheme. Australian Statistics on Medicines 1998. https://www.pbs.gov.au/info/statistics/asm/australian-statistics-on-medicines. Accessed 6 Jan 2022
Therapeutic Goods Administration. Medicines and vaccines post-market vigilance – statistics for 2017. https://www.tga.gov.au/resources/publication/publications/medicines-and-vaccines-post-market-vigilance-statistics-2017. Accessed 10 Jan 2023
Therapeutic Goods Administration. Half Yearly Performance Snapshot for July-December 2020. https://www.tga.gov.au/resourse/half-yearly-performance-snapshot-july-december-2020. Accessed 7 Jan 2022.
Therapeutic Goods Administration. Annual Performance Statistics Reports. https://www.tga.gov.au/annual-performance-statistics-reports. Accessed 7 Jan 2022.
Medicines and Healthcare products Regulatory Agency. Trends in UK spontaneous adverse drug reaction (ADR) reporting between 2008–2001. Archived content. https://www.nationalarchives.gov.uk. Accessed 8 Apr 2022.
Medicine and Healthcare products Regulatory Agency. Human Medicines Regulations Advisory Bodies Annual Report 2016;30. https://www.gov.uk/government/publications/human-medicines-regulations-2012-advisory-bodies-annual-report-2016. Accessed 24 Jun 2021.
Office for National Statistics. United Kingdom population mid-year estimate time series dataset (pop) 25 June 2021. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/timeseries/ukpop/pop. Accessed 23 May 2022.
Stats NZ. Estimated resident population of New Zealand as at June 2021. Available at: https://www.stats.govt.nz/information-releases/national-population-estimates-at-30-june-2021/. Accessed 6 Nov 2021.
Australian Bureau of Statistics. Estimated Resident Population; Persons; Australia Series ID A2060842F June-1981 to March 2021. www.abs.gov.au/statistics/people/population/national-state-and-territory-population/mar-2021#data-download. Accessed 23 May 2022.
National Library of Australia. TROVE. Available at: https://webarchive.nla.gov.au/awa/20210121072842/https://www.tga.gov.au/publication/australian-adverse-drug-reactions-bulletin. Accessed 3 Jul 2022.
Therapeutic Goods Administration. Medicines Safety Update. https://www.tga.gov.au/resources/publication/publications/medicines-safety-update. Accessed 3 Jul 2022.
McEwen J, Boyd IW. Near-death of Australia’s Medicines Safety Update. Intern Med J. 2021;51(7):1178–81. https://doi.org/10.1111/imj.15413.
Perry LT, Bhasale A, Fabbri A, Lexchin J, Puil L, Loarder M, et al. A descriptive analysis of medicines safety advisories issued by national medicines regulators in Australia, Canada, the United Kingdom and the United States – 2007 to 2016. Pharmacoepidemiol Drug Saf. 2020;29(9):1054–63. https://doi.org/10.1002/pds.5072.
Medicines and Healthcare products Regulatory Agency. Yellow Card: please help to reverse the decline in reporting of suspected adverse reactions. Drug Safety Update. 2019;12(10):4. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/802701/May-2019-PDF-DSU.pdf. Accessed 24 Jun 2021.
Roughead EE, Lexchin J. Adverse drug events: counting is not enough, action is needed. Med J Aust. 2006;184:315–6.
Li R, Curtain C, Bereznicki L, Zaidi STR. Community pharmacists’ knowledge and perspectives of reporting adverse drug reactions in Australia: a cross-sectional survey. Int J Clin Pharm. 2018;40(4):878–89. https://doi.org/10.1007/s11096-018-0700-2.
Martin JH, Lucas C. Reporting adverse drug events to the Therapeutic Goods Administration. Aust Prescr. 2021;44(1):2–3. https://doi.org/10.18773/austprescr.2020.077.
Australian Government Department of Health. Review of Medicines and Medical Devices Legislation, March 2015. https://webarchive.nla.gov.au/awa/20160620141320/https://www.health.gov.au/internet/main/publishing.nsf/Content/Expert-Review-of-Medicines-and-Medical-Devices-Regulation. Accessed 15 Apr 2022.
Moore TJ, Furberg CD, et al. Completeness of serious adverse drug event reports received by the US Food and Drug Administration in 2014. Pharmacoepidemiol Drug Saf. 2016;25(6):713–8. https://doi.org/10.1002/pds.3979.
Therapeutic Goods Administration. Pharmacovigilance Inspection Program metrics report: Jan–Dec 2020. https://www.tga.gov.au/resources/publication/publications/pharmacovigilance-inspection-program-metrics-report-jan-dec-2020. Accessed 15 Apr 2022.
Biriell C, Edwards IR. Reasons for reporting adverse drug reactions—some thoughts based on an international review. Pharmacoepidemiol Drug Saf. 1997;6(1):21–6. https://doi.org/10.1002/(SICI)1099-1557(199701)6:1%3c21::AID-PDS259%3e3.0.CO;2-I.
Acknowledgements
The authors would like to acknowledge the contributions of Dr Michael Tatley, Director, New Zealand Pharmacovigilance Centre, Dunedin, New Zealand, for the provision of yearly numbers of GP reporting in New Zealand; Phil Tregunno, Deputy Director, Patient Safety Monitoring, Safety and Surveillance, Medicines and Healthcare products Regulatory Agency, UK, for the provision of yearly numbers of GP reporting in the UK; and Dr Jane Cook, First Assistant Secretary, Medicines Regulation Division, Health Products Regulation Group, TGA, for the provision of yearly numbers of GP reporting in Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was used in the preparation of the paper.
Conflicts of Interest
As an employee of the Department of Health from 1986 to 2007, Ian Boyd had responsibility for the content and publication of Australian Adverse Drug Reactions Bulletins. As an employee of the Department of Health from 1979 to 1989 and from 1994 to 2005, John McEwen had involvement in the preparation and publication of Australian Adverse Drug Reactions Bulletins.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
All data generated or analysed during this study are included in this published article and its supplementary information.
Code Availability
Not applicable.
Author Contributions
Both authors contributed equally to this study, and both authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boyd, I.W., McEwen, J. Sustained Decline of Direct General Practitioner Reporting of Adverse Drug Reactions in Australia and Paucity in Details of Australian Reports in Safety Advisories. Drug Saf 46, 703–710 (2023). https://doi.org/10.1007/s40264-023-01321-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01321-4