Abstract
Introduction
Recently, cases of cardiovascular toxicities, such as pericarditis, caused by anaplastic lymphoma kinase (ALK) inhibitors have been reported; however, whether these adverse events are common among all ALK inhibitors remains unclear.
Aims
This study aimed to clarify the cardiovascular toxicity profile of ALK inhibitors using an adverse event spontaneous report database.
Methods
We analyzed data from VigiBase, the WHO global database of individual safety reports, from its inception in 1968 to December 2021. We calculated the reporting odds ratio to evaluate the association between ALK inhibitors (crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib) and 21 cardiovascular adverse events. Time to onset of pericarditis from ALK inhibitor administration was analyzed.
Results
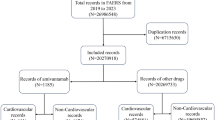
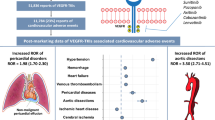
Of the 27,994,584 reports, 19,911 involved treatment with ALK inhibitors. Among the 21 cardiovascular toxicities, only pericarditis signals were detected with all five ALK inhibitors (crizotinib [reporting odds ratios (ROR), 4.7; 95% CI 3.63–6.15], ceritinib [ROR, 12.9; 95% CI 9.37–17.79], alectinib [ROR, 4.8; 95% CI 3.15–7.42], brigatinib [ROR, 3.5; 95% CI 1.33–9.46], and lorlatinib [ROR, 6.4; 95% CI 3.60–11.22]). For torsade de pointes/QT prolongation, signals were detected with crizotinib (ROR, 5.0; 95% CI 3.72–6.77) and ceritinib (ROR, 4.2; 95% CI 2.17–8.05), whereas for hypertension, they were identified only with brigatinib (ROR, 3.9; 95% CI 2.88–5.20), and for heart failure, they were detected with alectinib (ROR, 2.2; 95% CI 1.60–2.90), crizotinib (ROR, 2.1; 95% CI 1.72–2.48), and lorlatinib (ROR, 2.0; 95% CI 1.27–3.23). Regarding time-to-onset analysis from drug administration to adverse event reporting, for pericarditis, it ranged from 52.5 days for alectinib to 166.5 days for crizotinib.
Conclusions
Systematic evaluation of ALK inhibitor-associated adverse events revealed differences in the cardiotoxicity profiles among ALK inhibitors. Understanding the differences in the cardiovascular toxicity profile of each ALK inhibitor will contribute to safe drug therapy when switching between ALK inhibitors.



Similar content being viewed by others
References
Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006.
Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–55.
Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol. 2016;27:iii4-15.
Blackhall F, Ross Camidge D, Shaw AT, Soria J-C, Solomon BJ, Mok T, et al. Final results of the large-scale multinational trial PROFILE 1005: efficacy and safety of crizotinib in previously treated patients with advanced/metastatic ALK-positive non-small-cell lung cancer. ESMO Open. 2017;2: e000219.
Kim D-W, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016;17:452–63.
Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39.
Richly H, Kim TM, Schuler M, Kim D-W, Harrison SJ, Shaw AT, et al. Ceritinib in patients with advanced anaplastic lymphoma kinase–rearranged anaplastic large-cell lymphoma. Blood. 2015;126:1257–8.
Oyakawa T, Muraoka N, Iida K, Kusuhara M, Kawamura T, Naito T, et al. Crizotinib-induced simultaneous multiple cardiac toxicities. Invest New Drugs. 2018;36:949–51.
Imazio M, Gaita F, LeWinter M. Evaluation and treatment of pericarditis: a systematic review. JAMA. 2015;314:1498–506.
Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N Engl J Med. 2018;379:2027–39.
Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19:1654–67.
VigiBase. https://who-umc.org/vigibase/. Accessed 10 July 2022.
SQLite. https://www.sqlite.org/index.html. Accessed 10 Mar 2022.
Medical Dictionary for Regulatory Activities (MedDRA). https://www.meddra.org/how-to-use/support-documentation/english. Accessed 14 Mar 2022.
Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–89.
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77.
Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–29.
Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: Final results of phase 3 ALTA-1L trial. J Thorac Oncol. 2021;16:2091–108.
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–29.
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–23.
Lindquist M. The need for definitions in pharmacovigilance. Drug Saf. 2007;30:825–30.
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11:3–10.
Lin L, Zhao J, Kong N, He Y, Hu J, Huang F, et al. Meta-analysis of the incidence and risks of interstitial lung disease and QTc prolongation in non-small-cell lung cancer patients treated with ALK inhibitors. Oncotarget. 2017;8:57379–85.
Ulhoi MP, Sorensen BS, Meldgaard P. Alectinib-induced pleural and pericardial effusions in ALK-positive NSCLC. Case Rep Oncol. 2021;14:1323–7.
Costa RB, Costa RLB, Talamantes SM, Kaplan JB, Bhave MA, Rademaker A, et al. Systematic review and meta-analysis of selected toxicities of approved ALK inhibitors in metastatic non-small cell lung cancer. Oncotarget. 2018;9:22137–46.
Sullivan I, Planchard D. ALK inhibitors in non-small cell lung cancer: the latest evidence and developments. Ther Adv Med Oncol. 2016;8:32–47.
Mladěnka P, Applová L, Patočka J, Costa VM, Remiao F, Pourová J, et al. Comprehensive review of cardiovascular toxicity of drugs and related agents. Med Res Rev. 2018;38:1332–403.
XALKORI PHARMACOLOGY REVIEW, FDA, March 30 (2011), https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202570Orig1s000PharmR.pdf. Accessed 21 Sept 2022.
LORBRENA MULTI-DISCIPLINE REVIEW, FDA, December 5 (2017), https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210868Orig1s000MultidisciplineR.pdf. Accessed 21 Sept 2022.
Brenner AK, Gunnes MW. Therapeutic targeting of the anaplastic lymphoma kinase (ALK) in neuroblastoma-a comprehensive update. Pharmaceutics. 2021;13:1427.
Acknowledgments
The study results and conclusions do not represent the opinion of the Uppsala Monitoring Centre, National Centers, or WHO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by grants from the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research [Grant number: 21K20720, 22K15319] and Grant-in-Aid for Transformative Research Areas (B) [Grant number: 20H05798].
Conflicts of interest
The authors declare no potential conflicts of interest.
Ethics approval
Not applicable.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data that support the findings of this study are available from VigiBase, were used under license, and are, therefore, not publicly available. Data are available from VigiBase with the permission of Uppsala Monitoring Centre.
Code availability
The code used when analyzing the data of the current study is available from the corresponding author on reasonable request.
Author contributions
TN authored the article and performed the statistical analysis. HH conceptualized the study and was involved in planning and interpretation of results. KM, YN, HU, MY, SM, FA, KY, TK, MG, YK, YI, YZ, and KI critically revised the manuscript and interpreted the results. All authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niimura, T., Miyata, K., Hamano, H. et al. Cardiovascular Toxicities Associated with Anaplastic Lymphoma Kinase Inhibitors: A Disproportionality Analysis of the WHO Pharmacovigilance Database (VigiBase). Drug Saf 46, 545–552 (2023). https://doi.org/10.1007/s40264-023-01300-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01300-9