Abstract
Background
P2X3 receptor antagonists hold promising potential as a therapeutic option for patients with refractory or unexplained chronic cough, a condition lacking approved therapies. This study assessed the safety, tolerability, and pharmacokinetics (PK) of HRS-2261, a novel selective P2X3 receptor antagonist, in healthy subjects.
Methods
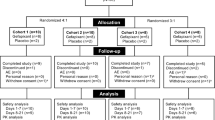
This randomized, double-blinded, placebo-controlled phase 1 trial of HRS-2261 consisted of three phases: the single ascending dose (SAD) study phase, the food-effect study phase, and the multiple ascending dose (MAD) study phase. In the SAD phase, healthy subjects were randomly assigned to receive a single oral dose of HRS-2261 (25, 100, 200, 400, 800, and 1200 mg) or placebo. Subjects in the 200 mg group of the SAD phase progressed directly to the food-effect phase following safety evaluation. In the MAD phase, healthy subjects were randomized to receive HRS-2261 (50, 200, and 400 mg) or placebo twice daily for 14 consecutive days. The primary endpoints were safety and tolerability.
Results
A total of 62 and 30 subjects were enrolled in the SAD and MAD phases, respectively, with 12 subjects from the SAD phase transitioning to the food-effect phase. The incidence and severity of adverse events (AEs) were not dose dependent, and most AEs were mild except for one moderate AE (epididymitis, which was not related to treatment) in the 400 mg group. Dysgeusia was reported in nine subjects, including two from the SAD phase, one from the food-effect phase, and six from the MAD phase. The median Tmax and geometric mean t1/2 were 0.9–2.0 h and 4.1–8.5 h in the SAD, and 2.0–2.7 h and 4.6–5.0 h on day 14 in the MAD, respectively. Drug exposures in the SAD and MAD phases were both less than dose proportional. The accumulation of the drug was slight with repeated twice-daily dosing. Food-effect study results showed that food intake did not affect the plasma exposure of HRS-2261.
Conclusions
HRS-2261 demonstrated good tolerability, with a low incidence of dysgeusia. The PK profile was favorable. This study supports further development of HRS-2261 as a potential P2X3 receptor antagonist for chronic cough.
Trial Registration Number
Clinical trials.gov, identifier: NCT05274516. Trial registration date: March 10, 2022.


Similar content being viewed by others
References
Chung KF, McGarvey L, Song WJ, Chang AB, Lai K, Canning BJ, Birring SS, Smith JA, Mazzone SB. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers. 2022;8(1):45.
Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, O’Connell F, Geppetti P, Gronke L, De Jongste J, et al. The diagnosis and management of chronic cough. Eur Respir J. 2004;24(3):481–92.
Smith JA, Woodcock A. Chronic cough. N Engl J Med. 2016;375(16):1544–51.
Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371(9621):1364–74.
Song WJ, Chang YS, Faruqi S, Kim JY, Kang MG, Kim S, Jo EJ, Kim MH, Plevkova J, Park HW, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479–81.
Won HK, Song WJ. Impact and disease burden of chronic cough. Asia Pac Allergy. 2021;11(2): e22.
Huang K, Gu X, Yang T, Xu J, Yang L, Zhao J, Zhang X, Bai C, Kang J, Ran P, et al. Prevalence and burden of chronic cough in China: a national cross-sectional study. ERJ Open Res. 2022;8(3):00075–2022.
Meltzer EO, Zeiger RS, Dicpinigaitis P, Bernstein JA, Oppenheimer JJ, Way NA, Li VW, Boggs R, Doane MJ, Urdaneta E, et al. Prevalence and burden of chronic cough in the United States. J Allergy Clin Immunol Pract. 2021;9(11):4037-4044 e4032.
Chung KF. Currently available cough suppressants for chronic cough. Lung. 2008;186(Suppl 1):S82-87.
Chung KF. Drugs to suppress cough. Expert Opin Investig Drugs. 2005;14(1):19–27.
Morice A, Dicpinigaitis P, McGarvey L, Birring SS. Chronic cough: new insights and future prospects. Eur Respir Rev. 2021;30(162):210127.
Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, Hilton Boon M, Kantar A, Lai K, McGarvey L, Rigau D, Satia I, Smith J, Song WJ, Tonia T, van den Berg JWK, van Manen MJG, Zacharasiewicz A. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55(1):1901136.
Sykes DL, Zhang M, Morice AH. Treatment of chronic cough: P2X3 receptor antagonists and beyond. Pharmacol Ther. 2022;237:108166.
McGarvey LP, Birring SS, Morice AH, Dicpinigaitis PV, Pavord ID, Schelfhout J, Nguyen AM, Li Q, Tzontcheva A, Iskold B, et al. Efficacy and safety of gefapixant, a P2X(3) receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. 2022;399(10328):909–23.
Smith JA, Kitt MM, Morice AH, Birring SS, McGarvey LP, Sher MR, Li YP, Wu WC, Xu ZJ, Muccino DR, et al. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med. 2020;8(8):775–85.
Markham A. Gefapixant: first approval. Drugs. 2022;82(6):691–5.
Hummel J, McKendrick S, Brindley C, French R. Exploratory assessment of dose proportionality: review of current approaches and proposal for a practical criterion. Pharm Stat. 2009;8(1):38–49.
Smith J, Mcgarvey L, Birring S, Morice A, Sher M, Dicpinigaitis P, Blaiss M, Lanouette S, Harvey L, Yang R, et al. Safety and efficacy of BLU-5937 in the treatment of refractory chronic cough from the Phase 2b Soothe Trial. American Thoracic Society Meeting. 2022:A5778-A5778.
Results of RELIEF, a phase 2a study with BLU-5937 in refractory chronic cough. ICS Meeting 2021. https://bellusdev.com/wp-content/uploads/ICS2021-Bellus-Health-RELIEF-trial-January-22-2021.pdf. Accessed 1 June 2023.
Nussbaum JC, Hussain A, Ma B, Min KC, Chen Q, Tomek C, Iwamoto M, Stoch SA. Characterization of the absorption, metabolism, excretion, and mass balance of gefapixant in humans. Pharmacol Res Perspect. 2022;10(1): e00924.
Chawla A, Largajolli A, Hussain A, Kleijn H, Ait-Oudhia S, Anton J, Krishna Ananthula H, Nussbaum J, La Rosa C, Gheyas F. Population pharmacokinetic analysis of the P2X3-receptor antagonist gefapixant. CPT Pharmacometr Syst Pharmacol. 2023;12(8):1107–18.
McCrea JB, Hussain A, Ma B, Garrett GC, Evers R, Laabs JE, Stoch SA, Iwamoto M. Assessment of pharmacokinetic interaction between gefapixant (MK-7264), a P2X3 receptor antagonist, and the OATP1B1 drug transporter substrate pitavastatin. Clin Pharmacol Drug Dev. 2022;11(3):406–12.
Garceau D, Chauret N, Harvey L. BLU-5937 a highly selective P2X3 homotrimeric receptor antagonist with improved taste safety profile in healthy subjects. American Thoracic Society Meeting. 2019:A7396–A7396.
Acknowledgements
We thank all participants and their families and acknowledge the contributions of all investigators in this trial. We would also like to acknowledge Tengfei Zhang (PhD, Medical Writer, Jiangsu Hengrui Pharmaceuticals Co., Ltd.) for medical writing support according to Good Publication Practice Guidelines.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Conflict of interest
XW, KS, ZH, and HW are employees of Jiangsu Hengrui Pharmaceuticals Co., Ltd. Other co-authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guideline. Study protocol and all amendments were approved by the independent ethics committee of The Second Affiliated Hospital of Anhui Medical University. All subjects provided written informed consent before enrollment.
Consent for publication
Not applicable.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
QianZ, WH, and HW were responsible for the conception and design of the study. YF, XZ, QinZ, LZ, RZ, CS, XW, ZH, HW, QianZ, and WH contributed to the data collection. KS was responsible for the statistical analysis. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Zhang, X., Zhang, Q. et al. Safety and Pharmacokinetics of HRS-2261, a P2X3 Receptor Antagonist, in Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled Phase 1 Study. Clin Pharmacokinet 63, 293–302 (2024). https://doi.org/10.1007/s40262-023-01330-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01330-7