Abstract
Background and Objective
Escitalopram and sertraline are commonly prescribed for anxiety and depressive disorders in children and adolescents. The pharmacokinetics (PK) of these medications have been evaluated in adults and demonstrate extensive variability, but studies in pediatric patients are limited. Therefore, we performed a population PK analysis for escitalopram and sertraline in children and adolescents to characterize the effects of demographic, clinical, and pharmacogenetic factors on drug exposure.
Methods
A PK dataset was generated by extracting data from the electronic health record and opportunistic sampling of escitalopram- and sertraline-treated psychiatrically hospitalized pediatric patients aged 5–18 years. A population PK analysis of escitalopram and sertraline was performed using NONMEM. Concentration-time profiles were simulated using MwPharm++ to evaluate how covariates included in the final models influence medication exposure and compared to adult therapeutic reference ranges.
Results
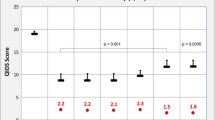
The final escitalopram cohort consisted of 315 samples from 288 patients, and the sertraline cohort consisted of 265 samples from 255 patients. A one-compartment model with a proportional residual error model best described the data for both medications. For escitalopram, CYP2C19 phenotype and concomitant CYP2C19 inhibitors affected apparent clearance (CL/F), and normalizing CL/F and apparent volume of distribution (V/F) to body surface area (BSA) improved estimations. The final escitalopram model estimated CL/F and V/F at 14.2 L/h/1.73 m2 and 428 L/1.73 m2, respectively. For sertraline, CYP2C19 phenotype and concomitant CYP2C19 inhibitors influenced CL/F, and empirical allometric scaling of patient body weight on CL/F and V/F was significant. The final sertraline model estimated CL/F and V/F at 124 L/h/70 kg and 4320 L/70 kg, respectively. Normalized trough concentrations (Ctrough) for CYP2C19 poor metabolizers taking escitalopram were 3.98-fold higher compared to normal metabolizers (151.1 ng/mL vs 38.0 ng/mL, p < 0.0001), and normalized Ctrough for CYP2C19 poor metabolizers taking sertraline were 3.23-fold higher compared to normal, rapid, and ultrarapid metabolizers combined (121.7 ng/mL vs 37.68 ng/mL, p < 0.0001). Escitalopram- and sertraline-treated poor metabolizers may benefit from a dose reduction of 50–75% and 25–50%, respectively, to normalize exposure to other phenotypes.
Conclusion
To our knowledge, this is the largest population PK analysis of escitalopram and sertraline in pediatric patients. Significant PK variability for both medications was observed and was largely explained by CYP2C19 phenotype. Slower CYP2C19 metabolizers taking escitalopram or sertraline may benefit from dose reductions given increased exposure.




Similar content being viewed by others
References
Hussain FS, Dobson ET, Strawn JR. Pharmacologic treatment of pediatric anxiety disorders. Curr Treat Options Psych. 2016;3:151–60.
Patel DR, Feucht C, Brown K, Ramsay J. Pharmacological treatment of anxiety disorders in children and adolescents: a review for practitioners. Transl Pediatr. 2018;7:23–55.
Dwyer JB, Bloch MH. Antidepressants for pediatric patients. Curr Psychiatr. 2019;18:26-42F.
Strawn JR, Mills JA, Schroeder H, Mossman SA, Varney ST, Ramsey LB, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81:20m1396.
Sakolsky DJ, Perel JM, Emslie GJ, Clarke GN, Wagner KD, Vitiello B, et al. Antidepressant exposure as a predictor of clinical outcomes in the Treatment of Resistant Depression in Adolescents (TORDIA) study. J Clin Psychopharmacol. 2011;31:92–7.
von Moltke LL, Greenblatt DJ, Grassi JM, Granda BW, Venkatakrishnan K, Duan SX, et al. Citalopram and desmethylcitalopram in vitro: human cytochromes mediating transformation, and cytochrome inhibitory effects. Biol Psychiat. 1999;46:839–49.
Obach RS, Cox LM, Tremaine LM. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos. 2005;33:262–70.
Hicks JK, Bishop JR, Sangkuhl K, Müller DJ, Ji Y, Leckband SG, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98:127–34.
Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017;19:215–23.
Chang M, Tybring G, Dahl M-L, Lindh JD. Impact of cytochrome P450 2C19 polymorphisms on citalopram/escitalopram exposure: a systematic review and meta-analysis. Clin Pharmacokinet. 2014;53:801–11.
Jukić MM, Haslemo T, Molden E, Ingelman-Sundberg M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: a retrospective study based on 2,087 patients. AJP. 2018;175:463–70.
Bråten LS, Haslemo T, Jukic MM, Ingelman-Sundberg M, Molden E, Kringen MK. Impact of CYP2C19 genotype on sertraline exposure in 1200 Scandinavian patients. Neuropsychopharmacology. 2020;45:570–6.
Rudberg I, Mohebi B, Hermann M, Refsum H, Molden E. Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharmacol Ther. 2008;83:322–7.
Wang J. Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin Pharmacol Ther. 2001;70:42–7.
Rudberg I, Hendset M, Uthus LH, Molden E, Refsum H. Heterozygous mutation in CYP2C19 significantly increases the concentration/dose ratio of racemic citalopram and escitalopram (S-citalopram). Ther Drug Monit. 2006;28:102–5.
Rudberg I, Hermann M, Refsum H, Molden E. Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. Eur J Clin Pharmacol. 2008;64:1181–8.
Allegaert K, den Anker J. Ontogeny of phase I metabolism of drugs. The J Clin Pharmacol [Internet]. 2019. https://doi.org/10.1002/jcph.1483.
Upreti VV, Wahlstrom JL. Meta-analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2016;56:266–83.
Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46:281–90.
Food and Drug Administration. Clinical Pharmacology/Biopharmaceutics Review of Three Pharmacokinetic Studies in Adolescent Children 12-17 Years for Lexapro. (2009) [Internet]. [cited 2023 Feb 3]. https://www.accessdata.fda.gov/drugsatfda_docs/pediatric/21323_Escitalopram_clinpharm_PREA.pdf. Accessed 3 Feb 2023.
Søgaard B, Mengel H, Rao N, Larsen F. The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J Clin Pharmacol. 2005;45:1400–6.
Alderman J, Wolkow R, Chung M, Johnston HF. Sertraline treatment of children and adolescents with obsessive-compulsive disorder or depression: pharmacokinetics, tolerability, and efficacy. J Am Acad Child Adolesc Psychiatry. 1998;37:386–94.
Axelson DA, Perel JM, Birmaher B, Rudolph GR, Nuss S, Bridge J, et al. Sertraline pharmacokinetics and dynamics in adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:1037–44.
Ronfeld RA, Tremaine LM, Wilner KD. Pharmacokinetics of sertraline and its N-demethyl metabolite in elderly and young male and female volunteers. Clin Pharmacokinet. 1997;32:22–30.
DeVane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41:1247–66.
Walsh TJ, Karlsson MO, Driscoll T, Arguedas AG, Adamson P, Saez-Llorens X, et al. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob Agents Chemother. 2004;48:2166–72.
Yanni SB, Annaert PP, Augustijns P, Ibrahim JG, Benjamin DK, Thakker DR. In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab Dispos. 2010;38:25–31.
Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44:195–235.
Bousman CA, Stevenson JM, Ramsey LB, Sangkuhl K, Hicks JK, Strawn JR, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin Pharmacol Ther. 2023;114(1):51–68.
Strawn JR, Mills JA, Poweleit EA, Ramsey LB, Croarkin PE. Adverse effects of antidepressant medications and their management in children and adolescents. Pharmacotherapy. 2023;43(7):675–90.
Van Driest SL, Marshall MD, Hachey B, Beck C, Crum K, Owen J, et al. Pragmatic pharmacology: population pharmacokinetic analysis of fentanyl using remnant samples from children after cardiac surgery: Population PK of fentanyl using remnant samples from children. Br J Clin Pharmacol. 2016;81:1165–74.
Tang Girdwood SC, Tang PH, Murphy ME, Chamberlain AR, Benken LA, Jones RL, et al. Demonstrating feasibility of an opportunistic sampling approach for pharmacokinetic studies of β-lactam antibiotics in critically ill children. J Clin Pharmacol. 2021;61:565–73.
Flockhart D, Thacker D, McDonald C, Desta Z. The Flockhart Cytochrome P450 Drug-Drug Interaction Table. Division of Clinical Pharmacology, Indiana University School of Medicine (Updated 2021) [Internet]. [cited 2023 Jan 26]. https://drug-interactions.medicine.iu.edu/. Accessed 26 Jan 2023.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95: 103208.
Liu S, Xiao T, Huang S, Li X, Kong W, Yang Y, et al. Population pharmacokinetics model for escitalopram in Chinese psychiatric patients: effect of CYP2C19 and age. Front Pharmacol. 2022;13: 964758.
Oliveira P, Ribeiro J, Donato H, Madeira N. Smoking and antidepressants pharmacokinetics: a systematic review. Ann Gen Psychiatry. 2017;16:17.
Scherf-Clavel M, Deckert J, Menke A, Unterecker S. Smoking Is associated with lower dose-corrected serum concentrations of escitalopram. J Clin Psychopharmacol. 2019;39:485–8.
Ramsey LB, Prows CA, Chidambaran V, Sadhasivam S, Quinn CT, Teusink-Cross A, et al. Implementation of CYP2D6-guided opioid therapy at Cincinnati Children’s Hospital Medical Center. Am J Health-Syst Pharm. 2023;80(13):852–59.
Ramsey LB, Prows CA, Zhang K, Saldaña SN, Sorter MT, Pestian JP, et al. Implementation of pharmacogenetics at cincinnati children’s hospital medical center: lessons learned over 14 years of personalizing medicine. Clin Pharmacol Ther. 2019;105:49–52.
Caudle KE, Sangkuhl K, Whirl-Carrillo M, Swen JJ, Haidar CE, Klein TE, et al. Standardizing CYP2D6 genotype to phenotype translation: consensus recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin Transl Sci. 2020;13:116–24.
Bauer RJ. NONMEM tutorial part I: description of commands and options, with simple examples of population analysis. CPT Pharmacometrics Syst Pharmacol. 2019;8:525–37.
Mould D, Upton R. Basic concepts in population modeling, simulation, and model-based drug development. CPT Pharmacomet Syst Pharmacol. 2012;1:6.
Courlet P, Guidi M, Glatard A, Alves Saldanha S, Cavassini M, Buclin T, et al. Escitalopram population pharmacokinetics in people living with human immunodeficiency virus and in the psychiatric population: Drug–drug interactions and probability of target attainment. Br J Clin Pharmacol. 2019;85:2022–32.
Lexapro [Package Insert] [Internet]. Allergan, Inc. 2021 [cited 2023 Jan 26]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13bb8267-1cab-43e5-acae-55a4d957630a. Accessed 26 Jan 2023.
Zoloft [Package Insert] [Internet]. Roerig. 2021 [cited 2023 Jan 26]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fe9e8b7d-61ea-409d-84aa-3ebd79a046b5. Accessed 26 Jan 2023.
Dubois D, Dubois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;XVII(6_2):863–71.
Claudio-Campos K, Duconge J, Cadilla CL, Ruaño G. Pharmacogenetics of drug-metabolizing enzymes in US Hispanics. Drug Metab Pers Therapy [Internet]. 2015. https://doi.org/10.1515/dmdi-2014-0023/html.
Koopmans AB, Braakman MH, Vinkers DJ, Hoek HW, Van Harten PN. Meta-analysis of probability estimates of worldwide variation of CYP2D6 and CYP2C19. Transl Psychiatry. 2021;11:141.
Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:557–64.
Varigonda AL, Jakubovski E, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2016;55:851-859.e2.
Strawn JR, Mills JA, Sauley BA, Welge JA. The impact of antidepressant dose and class on treatment response in pediatric anxiety disorders: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2018;57:235-244.e2.
Jin Y, Pollock BG, Frank E, Florian J, Kirshner M, Fagiolini A, et al. The effect of reporting methods for dosing times on the estimation of pharmacokinetic parameters of escitalopram. J Clin Pharmacol. 2009;49:176–84.
Akil A, Bies RR, Pollock BG, Avramopoulos D, Devanand DP, Mintzer JE, et al. A population pharmacokinetic model for R- and S-citalopram and desmethylcitalopram in Alzheimer’s disease patients with agitation. J Pharmacokinet Pharmacodyn. 2016;43:99–109.
Jin Y, Pollock BG, Frank E, Cassano GB, Rucci P, Müller DJ, et al. Effect of Age, Weight, and CYP2C19 Genotype on Escitalopram Exposure. J Clin Pharmacol. 2010;50:62–72.
Jeppesen U, Gram LF, Vistisen K, Loft S, Poulsen HE, Brøsen K. Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol. 1996;51:73–8.
Harvey AT, Preskorn SH. Fluoxetine pharmacokinetics and effect on CYP2C19 in young and elderly volunteers. J Clin Psychopharmacol. 2001;21:161–6.
Carlsson B, Olsson G, Reis M, Walinder J, Nordin C, Lundmark J, et al. Enantioselective analysis of citalopram and metabolites in adolescents. Ther Drug Monit. 2001;23:658–64.
Pedersen RS, Noehr-Jensen L, Brosen K. The inhibitory effect of oral contraceptives on CYP2C19 activity is not significant in carriers of the CYP2C19*17 allele. Clin Exp Pharmacol Physiol. 2013;40(10):683–8.
Vaughn SE, Strawn JR, Poweleit EA, Sarangdhar M, Ramsey LB. The impact of marijuana on antidepressant treatment in adolescents: clinical and pharmacologic considerations. J Pers Med. 2021;11:615.
Zendulka O, Dovrtělová G, Nosková K, Turjap M, Šulcová A, Hanuš L, et al. Cannabinoids and cytochrome P450 interactions. CDM. 2016;17:206–26.
Saiz-Rodríguez M, Belmonte C, Román M, Ochoa D, Koller D, Talegón M, et al. Effect of polymorphisms on the pharmacokinetics, pharmacodynamics and safety of sertraline in healthy volunteers. Basic Clin Pharmacol Toxicol. 2018;122:501–11.
Hanan NJ, Paul ME, Huo Y, Kapetanovic S, Smith E, Siberry G, et al. Sertraline pharmacokinetics in hiv-infected and uninfected children, adolescents, and young adults. Front Pediatr. 2019;7:16.
Ishizawa Y, Yasui-Furukori N, Takahata T, Sasaki M, Tateishi T. The effect of aging on the relationship between the cytochrome P450 2C19 genotype and omeprazole pharmacokinetics. Clin Pharmacokinet. 2005;44:1179–89.
Li CH, Pollock BG, Lyketsos CG, Vaidya V, Drye LT, Kirshner M, et al. Population pharmacokinetic modeling of sertraline treatment in patients with Alzheimer disease: the DIADS-2 Study. J Clin Pharmacol. 2013;53:234–9.
Yuce-Artun N, Baskak B, Ozel-Kizil ET, Ozdemir H, Uckun Z, Devrimci-Ozguven H, et al. Influence of CYP2B6 and CYP2C19 polymorphisms on sertraline metabolism in major depression patients. Int J Clin Pharm. 2016;38:388–94.
Poweleit EA, Aldrich SL, Martin LJ, Hahn D, Strawn JR, Ramsey LB. Pharmacogenetics of sertraline tolerability and response in pediatric anxiety and depressive disorders. J Child Adolesc Psychopharmacol. 2019;29:348–61.
Gjestad C, Westin AA, Skogvoll E, Spigset O. Effect of proton pump inhibitors on the serum concentrations of the selective serotonin reuptake inhibitors citalopram, escitalopram, and sertraline. Ther Drug Monit. 2015;37:90–7.
Alhadab AA, Brundage RC. Population pharmacokinetics of sertraline in healthy subjects: a model-based meta-analysis. AAPS J. 2020;22:73.
Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246–51.
Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 metabolizer status on escitalopram/citalopram tolerability and response in youth with anxiety and depressive disorders. Front Pharmacol. 2019;10:99.
Eichentopf L, Hiemke C, Conca A, Engelmann J, Gerlach M, Havemann-Reinecke U, et al. Systematic review and meta-analysis on the therapeutic reference range for escitalopram: blood concentrations, clinical effects and serotonin transporter occupancy. Front Psychiatry. 2022;13: 972141.
Strawn JR, Dobson ET, Mills JA, Cornwall GJ, Sakolsky D, Birmaher B, et al. Placebo response in pediatric anxiety disorders: results from the child/adolescent anxiety multimodal study. J Child Adolesc Psychopharmacol. 2017;27:501–8.
Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11:558–69.
Beaulieu-Jones BK, Finlayson SG, Yuan W, Altman RB, Kohane IS, Prasad V, et al. Examining the use of real-world evidence in the regulatory process. Clin Pharmacol Ther. 2020;107:843–52.
Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–66.
Liu W, Li G, Wang C, Wang X, Yang L. Efficacy of sertraline combined with cognitive behavioral therapy for adolescent depression: a systematic review and meta-analysis. Comput Math Methods Med. 2021;2021:5309588.
Strawn JR, Mills JA, Suresh V, Peris TS, Walkup JT, Croarkin PE. Combining selective serotonin reuptake inhibitors and cognitive behavioral therapy in youth with depression and anxiety. J Affect Disord. 2022;298:292–300.
Strawn JR, Welge JA, Wehry AM, Keeshin BR, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32:149–57.
Stancil SL, Tumberger J, Strawn JR. Target to treatment: a charge to develop biomarkers of response and tolerability in child and adolescent psychiatry. Clin Transl Sci. 2022;15:816–23.
Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15:473–500.
Acknowledgments
We thank Josh Courter for helping set up the Vigilanz Alert System, the CCHMC Clinical Laboratories for sample collection, and Ashley Sarbell, Jada Bouyer, and Kynnedi Williams for data collection. We acknowledge the Center for Clinical & Translational Science and Training (CCTST) at the University of Cincinnati supports REDCap and is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant UL1TR001425. The CTSA program is led by the NIH's National Center for Advancing Translational Sciences (NCATS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures/Conflicts of Interest
LBR has received research support from BTG International. JRS has received research support from Abbvie, the National Institutes of Health, PCORI, the Yung Family Foundation, and receives material support from Myriad. He receives royalties from UpToDate and Cambridge and has consulted with Cerevel, Otsuka, and Intracellular Therapeutics. All other authors declared no competing interests for this work.
Funding Information
Funding from the Center for Pediatric Genomics at Cincinnati Children’s Hospital Medical Center supported this project. EAP is supported by the National Institute of Mental Health of the National Institutes of Health under award number F31MH132265. ZLT was supported by the National Institute of Child Health and Development T32 Cincinnati Pediatric Clinical Pharmacology Training Program (T32HD069054). ZD is supported by R35GM145383 from NIGMS. JRS, EAP, and LBR are supported by NICHD (R01HD099775).
Data Availability
Data for this study may be available upon reasonable request from the corresponding author.
Ethics Approval
This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center and was determined to be exempt in accordance with applicable regulations and institutional policy.
Consent to Participate
A waiver of informed consent was granted by the IRB at Cincinnati Children’s Hospital Medical Center.
Consent for Publication
Not applicable.
Code Availability
The code generated for the analysis may be available upon reasonable request from the corresponding author.
Author Contributions
All authors contributed to the study conception and design. Data collection was performed by EAP, SEV, and ZD. Data analysis was performed by EAP, ZLT, and TM. The first draft of the manuscript was written by EAP and all authors edited and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poweleit, E.A., Taylor, Z.L., Mizuno, T. et al. Escitalopram and Sertraline Population Pharmacokinetic Analysis in Pediatric Patients. Clin Pharmacokinet 62, 1621–1637 (2023). https://doi.org/10.1007/s40262-023-01294-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01294-8