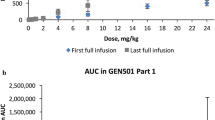
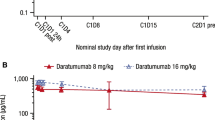
Abstract
Daratumumab is a fully human, monoclonal immunoglobulin G1 and a first-in-class CD38-targeting drug approved by the US Food and Drug Administration for the treatment of patients with relapsed/refractory and newly diagnosed multiple myeloma or newly diagnosed light-chain amyloidosis. CD38 is heavily expressed on malignant myeloma cells, and daratumumab exerts anti-myeloma activity via immune-mediated mechanisms, direct induction of apoptosis, and immunomodulation. Daratumumab is used as monotherapy or in combination with standard-of-care myeloma therapies, including proteasome inhibitors, immunomodulatory agents, DNA-alkylating agents, and corticosteroids. Following an intravenous infusion, daratumumab exhibits nonlinear pharmacokinetics (PK), as clearance decreases with higher doses and over time because of target-mediated effects. Dosing schedules vary depending on indications and co-administered drugs, but generally daratumumab is administered weekly for 6–9 weeks followed by a less frequent dosing regimen, once every 2–4 weeks. Daratumumab exposure is strongly correlated with efficacy, and the exposure–efficacy relationship follows a maximal effect model, whereas exposure is not correlated with safety endpoints. The approved dose of 16 mg/kg of daratumumab results in the saturation of 99% of the target at the end of weekly dosing in most patients, and high target saturation is maintained over time during the less frequent dosing schedule. Infusion-related reactions are frequently observed in patients given daratumumab, particularly with the first infusion, thus prompting long durations of infusion (~ 7 h) and splitting of the first dose across 2 days. This led to the development of a subcutaneous delivery formulation for daratumumab (Dara-SC). Dara-SC provides a similar efficacy and safety profile to intravenous daratumumab (Dara-IV) but has a much lower rate of infusion-related reactions and a shorter infusion time. Exposure–response relationships for efficacy and safety endpoints were similar between Dara-SC and Dara-IV, and co-administered drugs with either Dara-IV or Dara-SC do not significantly affect daratumumab PK. Except for baseline myeloma type and albumin level, none of the other investigated disease and patient characteristics (renal/hepatic function, age, sex, race, weight, Eastern Cooperative Oncology Group performance status) was identified to have clinically relevant effects on exposure to daratumumab monotherapy or combination therapy regimens. Dara-IV exposure was significantly lower in patients with immunoglobulin G myeloma compared with patients with non-immunoglobulin G myeloma (p < 0.0001) and in patients with a lower albumin level, whereas the overall response rate was similar regardless of the myeloma type and albumin level. Daratumumab dose adjustment is not currently recommended based on disease and patient characteristics.

Similar content being viewed by others
References
Offidani M, Corvatta L, More S, Nappi D, Martinelli G, Olivieri A, et al. Daratumumab for the management of newly diagnosed and relapsed/refractory multiple myeloma: current and emerging treatments. Front Oncol. 2020;10:624661. https://doi.org/10.3389/fonc.2020.624661.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Kumar SK, Dimopoulos MA, Kastritis E, Terpos E, Nahi H, Goldschmidt H, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. 2017;31(11):2443–8. https://doi.org/10.1038/leu.2017.138.
US Food and Drug Administration (FDA). Center for Drug Evaluation and Research. FDA letter for BLA 761036. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/761036Orig1s000ltr.pdf. Accessed 13 Oct 2022.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–31. https://doi.org/10.1056/NEJMoa1607751.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–66. https://doi.org/10.1056/NEJMoa1606038.
Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974–81. https://doi.org/10.1182/blood-2017-05-785246.
Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186–97. https://doi.org/10.1016/S0140-6736(20)30734-0.
US Food and Drug Administration (FDA). Darzalex (daratumumab) injection, for intravenous use: prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761036s041lbl.pdf. Accessed 13 Oct 2022.
US National Library of Medicine. Daratumumab after stem cell transplant in treating patients with multiple myeloma. Identifier NCT03346135. July 2019. https://clinicaltrials.gov/ct2/show/NCT03346135. Accessed 13 Oct 2022.
Cohen YC, Oriol A, Wu KL, Lavi N, Vlummens P, Jackson C, et al. Daratumumab with cetrelimab, an anti-PD-1 monoclonal antibody, in relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2021;21(1):46-54.e4. https://doi.org/10.1016/j.clml.2020.08.008.
Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88(3):841–86. https://doi.org/10.1152/physrev.00035.2007.
Malavasi F, Funaro A, Roggero S, Horenstein A, Calosso L, Mehta K. Human Cd38: a glycoprotein in search of a function. Immunol Today. 1994;15(3):95–7. https://doi.org/10.1016/0167-5699(94)90148-1.
Piedra-Quintero ZL, Wilson Z, Nava P, Guerau-de-Arellano M. CD38: an immunomodulatory molecule in inflammation and autoimmunity. Front Immunol. 2020;11:597959. https://doi.org/10.3389/fimmu.2020.597959.
Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121(4):482–8. https://doi.org/10.1039/74r4tb90buwh27jx.
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207–19. https://doi.org/10.1056/NEJMoa1506348.
de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8. https://doi.org/10.4049/jimmunol.1003032.
Overdijk MB, Verploegen S, Bogels M, van Egmond M, van Bueren JJL, Mutis T, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7(2):311–20. https://doi.org/10.1080/19420862.2015.1007813.
Gao Y, Li L, Zheng Y, Zhang W, Niu B, Li Y. Monoclonal antibody Daratumumab promotes macrophage-mediated anti-myeloma phagocytic activity via engaging FC gamma receptor and activation of macrophages. Mol Cell Biochem. 2022;477(8):2015–24. https://doi.org/10.1007/s11010-022-04390-8.
Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. J Immunol. 2016;197(3):807–13. https://doi.org/10.4049/jimmunol.1501351.
Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94. https://doi.org/10.1182/blood-2015-12-687749.
Adams HC, Stevenaert F, Krejcik J, Van der Borght K, Smets T, Bald J, et al. High-parameter mass cytometry evaluation of relapsed/refractory multiple myeloma patients treated with daratumumab demonstrates immune modulation as a novel mechanism of action. Cytom Part A. 2019;95a(3):279–89. https://doi.org/10.1002/cyto.a.23693.
Costa F, Toscani D, Chillemi A, Quarona V, Bolzoni M, Marchica V, et al. Expression of CD38 in myeloma bone niche: a rational basis for the use of anti-CD38 immunotherapy to inhibit osteoclast formation. Oncotarget. 2017;8(34):56598–611. https://doi.org/10.18632/oncotarget.17896.
Horenstein AL, Chillemi A, Quarona V, Zito A, Roato I, Morandi F, et al. NAD(+)-metabolizing ectoenzymes in remodeling tumor-host interactions: the human myeloma model. Cells-Basel. 2015;4(3):520–37. https://doi.org/10.3390/cells4030520.
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–60. https://doi.org/10.1016/S0140-6736(15)01120-4.
Clemens PL, Yan X, Lokhorst HM, Lonial S, Losic N, Khan I, et al. Pharmacokinetics of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitor and immunomodulatory drug treatment. Clin Pharmacokinet. 2017;56(8):915–24. https://doi.org/10.1007/s40262-016-0477-1.
Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–88. https://doi.org/10.1002/psp4.12224.
Yan XY, Clemens PL, Puchalski T, Lonial S, Lokhorst HM, Orlowski RZ, et al. Target-mediated drug disposition of daratumumab following intravenous infusion in relapsed or refractory multiple myeloma after prior proteasome inhibitors and immunomodulatory drugs: a population pharmacokinetic analysis. Blood. 2015. https://doi.org/10.1182/blood.V126.23.4222.4222.
Xu XS, Yan X, Puchalski T, Lonial S, Lokhorst HM, Voorhees PM, et al. Clinical implications of complex pharmacokinetics for daratumumab dose regimen in patients with relapsed/refractory multiple myeloma. Clin Pharmacol Ther. 2017;101(6):721–4. https://doi.org/10.1002/cpt.577.
Jacobs JFM, Mould DR. The role of FcRn in the pharmacokinetics of biologics in patients with multiple myeloma. Clin Pharmacol Ther. 2017;102(6):903–4. https://doi.org/10.1002/cpt.665.
Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J Immunol. 2015;194(10):4595–603. https://doi.org/10.4049/jimmunol.1403014.
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. https://doi.org/10.4065/78.1.21.
Landgren O, Hofmann JN, McShane CM, Santo L, Hultcrantz M, Korde N, et al. Association of immune marker changes with progression of monoclonal gammopathy of undetermined significance to multiple myeloma. JAMA Oncol. 2019;5(9):1293–301. https://doi.org/10.1001/jamaoncol.2019.1568.
Xu XS, Schecter JM, Jansson R, Yan X. Response to “The Role of FcRn in the Pharmacokinetics of Biologics in Patients with Multiple Myeloma.” Clin Pharmacol Ther. 2017;102(6):905. https://doi.org/10.1002/cpt.779.
Yan X, Clemens PL, Puchalski T, Lonial S, Lokhorst H, Voorhees PM, et al. Influence of disease and patient characteristics on daratumumab exposure and clinical outcomes in relapsed or refractory multiple myeloma. Clin Pharmacokinet. 2018;57(4):529–38. https://doi.org/10.1007/s40262-017-0598-1.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–5. https://doi.org/10.1182/blood-2010-10-299487.
Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37–44. https://doi.org/10.1182/blood-2016-03-705210.
Usmani SZ, Nahi H, Plesner T, Weiss BM, Bahlis NJ, Belch A, et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: final results from the phase 2 GEN501 and SIRIUS trials. Lancet Haematol. 2020;7(6):e447–55. https://doi.org/10.1016/S2352-3026(20)30081-8.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–59. https://doi.org/10.2165/11535960-000000000-00000.
Xu XS, Dimopoulos MA, Sonneveld P, Ho PJ, Belch A, Leiba M, et al. Pharmacokinetics and exposure-response analyses of daratumumab in combination therapy regimens for patients with multiple myeloma. Adv Ther. 2018;35(11):1859–72. https://doi.org/10.1007/s12325-018-0815-9.
Iida S, Suzuki K, Kusumoto S, Ri M, Tsukada N, Abe Y, et al. Safety and efficacy of daratumumab in Japanese patients with relapsed or refractory multiple myeloma: a multicenter, phase 1, dose-escalation study. Int J Hematol. 2017;106(4):541–51. https://doi.org/10.1007/s12185-017-2281-6.
Luo MM, Zhu PP, Nnane I, Xiong Y, Merlini G, Comenzo RL, et al. Population pharmacokinetics and exposure-response modeling of daratumumab subcutaneous administration in patients with light-chain amyloidosis. J Clin Pharmacol. 2022;62(5):656–69. https://doi.org/10.1002/jcph.1994.
White TA, Johnson S, Walseth TF, Lee HC, Graeff RM, Munshi CB, et al. Subcellular localization of cyclic ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities in porcine airway smooth muscle. Biochim Biophys Acta. 2000;1498(1):64–71. https://doi.org/10.1016/s0167-4889(00)00077-x.
Chari A, Martinez-Lopez J, Mateos MV, Blade J, Benboubker L, Oriol A, et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood. 2019;134(5):421–31. https://doi.org/10.1182/blood.2019000722.
Chari A, Usmani SZ, Krishnan A, Lonial S, Comenzo R, Wu KD, et al. Daratumumab (DARA) in combination with carfilzomib, lenalidomide, and dexamethasone (KRd) in patients with newly diagnosed multiple myeloma (MMY1001): updated results from an open-label, phase 1b study. Blood. 2017;7:130. https://doi.org/10.1182/blood.V130.Suppl_1.3110.3110.
Xu XS, Moreau P, Usmani SZ, Lonial S, Jakubowiak A, Oriol A, et al. Split first dose administration of intravenous daratumumab for the treatment of multiple myeloma (MM): clinical and population pharmacokinetic analyses. Adv Ther. 2020;37(4):1464–78. https://doi.org/10.1007/s12325-020-01247-8.
Rifkin R, Singer D, Aguilar KM, Baidoo B, Maiese EM. Daratumumab split first versus single dosing schedule among patients with multiple myeloma treated in a US community oncology setting: a retrospective observational study. Clin Ther. 2019;41(5):866–81. https://doi.org/10.1016/j.clinthera.2019.03.013.
US Food and Drug Administration (FDA). DARZALEX FASPRO (daratumumab and hyaluronidase-fihj) injection, for subcutaneous use: prescribing information. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761145s012lbl.pdf. Accessed 13 Oct 2022.
Barr H, Dempsey J, Waller A, Huang Y, Williams N, Sharma N, et al. Ninety-minute daratumumab infusion is safe in multiple myeloma. Leukemia. 2018;32(11):2495–518. https://doi.org/10.1038/s41375-018-0120-2.
Usmani SZ, Nahi H, Mateos MV, van de Donk N, Chari A, Kaufman JL, et al. Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma. Blood. 2019;134(8):668–77. https://doi.org/10.1182/blood.2019000667.
Paul B, Hamadeh I, Atrash S, Bhutani M, Voorhees P, Usmani SZ. Daratumumab subcutaneous formulation for the treatment of multiple myeloma. Expert Opin Biol Ther. 2020;20(11):1253–9. https://doi.org/10.1080/14712598.2020.1806231.
San-Miguel J, Usmani SZ, Mateos MV, van de Donk N, Kaufman JL, Moreau P, et al. Subcutaneous daratumumab in patients with relapsed or refractory multiple myeloma: part 2 of the open-label, multicenter, dose-escalation phase 1b study (PAVO). Haematologica. 2021;106(6):1725–32. https://doi.org/10.3324/haematol.2019.243790.
Clemens PL, Xu S, Luo M, Chari A, Usmani SZ, Mateos MV, et al. Pharmacokinetics (PK) of subcutaneous daratumumab in patients with relapsed or refractory (RR) multiple myeloma (MM): primary clinical pharmacology analysis of the open-label, multicenter, phase 1b study (PAVO). Blood. 2018;29:132. https://doi.org/10.1182/blood-2018-99-113161.
Mateos MV, Nahi H, Legiec W, Grosicki S, Vorobyev V, Spicka I, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): a multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020;7(5):e370–80. https://doi.org/10.1016/S2352-3026(20)30070-3.
Luo MM, Usmani SZ, Mateos MV, Nahi H, Chari A, San-Miguel J, et al. Exposure-response and population pharmacokinetic analyses of a novel subcutaneous formulation of daratumumab administered to multiple myeloma patients. J Clin Pharmacol. 2021;61(5):614–27. https://doi.org/10.1002/jcph.1771.
Chari A, Rodriguez-Otero P, McCarthy H, Suzuki K, Hungria V, Sureda Balari A, et al. Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy (PLEIADES): an open-label phase II study. Br J Haematol. 2021;192(5):869–78. https://doi.org/10.1111/bjh.16980.
Moreau P, Chari A, Haenel M, Oriol A, Rodriguez-Otero P, McCarthy H, et al. Subcutaneous daratumumab (DARA SC) plus standard-of-care (SoC) regimens in multiple myeloma (MM) across lines of therapy in the phase 2 Pleiades Study: initial results of the Dara SC plus carfilzomib/dexamethasone (D-Kd) cohort, and updated results for the Dara SC plus bortezomib/melphalan/rrednisone (D-VMP) and Dara SC plus lenalidomide/dexamethasone (D-Rd) cohorts. Blood. 2020;5:136. https://doi.org/10.1182/blood-2020-134935.
Dimopoulos MA, Terpos E, Boccadoro M, Delimpasi S, Beksac M, Katodritou E, et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(6):801–12. https://doi.org/10.1016/S1470-2045(21)00128-5.
Bahlis NJ, Dimopoulos MA, White DJ, Benboubker L, Cook G, Leiba M, et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020;34(7):1875–84. https://doi.org/10.1038/s41375-020-0711-6.
Kim JE, Yoo C, Lee DH, Kim SW, Lee JS, Suh C. Serum albumin level is a significant prognostic factor reflecting disease severity in symptomatic multiple myeloma. Ann Hematol. 2010;89(4):391–7. https://doi.org/10.1007/s00277-009-0841-4.
Chen JH, Hsu SN, Huang TC, Wu YY, Lin C, Chang PY, et al. Prognostic significance of initial serum albumin and 24 hour daily protein excretion before treatment in multiple myeloma. PLoS ONE. 2015;10(6):e0128905. https://doi.org/10.1371/journal.pone.0128905.
Greipp PR, San Miguel J, Durie BGM, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–20. https://doi.org/10.1200/Jco.2005.04.242.
Hussain A, Almenfi HF, Almehdewi AM, Hamza MS, Bhat MS, Vijayashankar NP. Laboratory features of newly diagnosed multiple myeloma patients. Cureus. 2019;11(5):e4716. https://doi.org/10.7759/cureus.4716.
Yadav P, Cook M, Cockwell P. Current trends of renal impairment in multiple myeloma. Kidney Dis (Basel). 2016;1(4):241–57. https://doi.org/10.1159/000442511.
Kuzume A, Tabata R, Terao T, Tsushima T, Miura D, Narita K, et al. Safety and efficacy of daratumumab in patients with multiple myeloma and severe renal failure. Br J Haematol. 2021;193(4):e33–6. https://doi.org/10.1111/bjh.17412.
Smyth E, Glavey S, Melotti D, Thornton P, Sargent J, Conlon P, et al. Dialysis independence following single-agent daratumumab in refractory myeloma with renal failure. Ir J Med Sci. 2019;188(3):1079–80. https://doi.org/10.1007/s11845-018-1951-6.
Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med. 2019;380(22):2104–15. https://doi.org/10.1056/NEJMoa1817249.
Gil-Sierra MD, Briceno-Casado MDP, Fenix-Caballero S, Alegre-Del Rey EJ, de la Lastra-Romero CA, Sanchez-Hidalgo M. Daratumumab-based therapies in transplant-ineligible patients with untreated multiple myeloma and hepatic dysfunction: a systematic review of subgroup analyses. J Oncol Pharm Pract. 2021;30:10781552211062144. https://doi.org/10.1177/10781552211062144.
Rah SY, Kim UH. CD38-mediated Ca(2+) signaling contributes to glucagon-induced hepatic gluconeogenesis. Sci Rep. 2015;3(5):10741. https://doi.org/10.1038/srep10741.
Xie L, Wen K, Li Q, Huang CC, Zhao JL, Zhao QH, et al. CD38 deficiency protects mice from high fat diet-induced nonalcoholic fatty liver disease through activating NAD(+)/sirtuins signaling pathways-mediated inhibition of lipid accumulation and oxidative stress in hepatocytes. Int J Biol Sci. 2021;17(15):4305–15. https://doi.org/10.7150/ijbs.65588.
Zhu J, Lu J, Tung HC, Liu K, Li J, Grant DM, et al. Cell type-specific roles of CD38 in the interactions of isoniazid with NAD(+) in the liver. Drug Metab Dispos. 2020;48(12):1372–9. https://doi.org/10.1124/dmd.120.000139.
Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–28. https://doi.org/10.1056/NEJMoa1714678.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29–38. https://doi.org/10.1016/S0140-6736(19)31240-1.
Landgren O, Weisel K, Rosinol L, Touzeau C, Turgut M, Hajek R, et al. Subgroup analysis based on cytogenetic risk in patients with relapsed or refractory multiple myeloma in the CANDOR study. Br J Haematol. 2022;198(6):988–93. https://doi.org/10.1111/bjh.18233.
Weisel K, Spencer A, Lentzsch S, Avet-Loiseau H, Mark TM, Spicka I, et al. Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: subgroup analysis of CASTOR based on cytogenetic risk. J Hematol Oncol. 2020;13(1):115. https://doi.org/10.1186/s13045-020-00948-5.
Kaufman JL, Dimopoulos MA, White D, Benboubker L, Cook G, Leiba M, et al. Daratumumab, lenalidomide, and dexamethasone in relapsed/refractory myeloma: a cytogenetic subgroup analysis of POLLUX. Blood Cancer J. 2020;10(11):111. https://doi.org/10.1038/s41408-020-00375-2.
Chong LL, Soon YY, Soekojo CY, Ooi M, Chng WJ, de Mel S. Daratumumab-based induction therapy for multiple myeloma: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;159:103211. https://doi.org/10.1016/j.critrevonc.2020.103211.
Premkumar V, Pan S, Lentzsch S, Bhutani D. Use of daratumumab in high risk multiple myeloma: a meta-analysis. eJHaem. 2020;1(1):267–71. https://doi.org/10.1002/jha2.47.
Giri S, Grimshaw A, Bal S, Godby K, Kharel P, Djulbegovic B, et al. Evaluation of daratumumab for the treatment of multiple myeloma in patients with high-risk cytogenetic factors: a systematic review and meta-analysis. JAMA Oncol. 2020;6(11):1759–65. https://doi.org/10.1001/jamaoncol.2020.4338.
Jakubowiak AJ, Kumar S, Medhekar R, Pei H, Lefebvre P, Kaila S, et al. Daratumumab improves depth of response and progression-free survival in transplant-ineligible, high-risk, newly diagnosed multiple myeloma. Oncologist. 2022;27(7):e589–96. https://doi.org/10.1093/oncolo/oyac067.
Suzuki K, Dimopoulos MA, Takezako N, Okamoto S, Shinagawa A, Matsumoto M, et al. Daratumumab, lenalidomide, and dexamethasone in East Asian patients with relapsed or refractory multiple myeloma: subgroup analyses of the phase 3 POLLUX study. Blood Cancer J. 2018;8(4):41. https://doi.org/10.1038/s41408-018-0071-x.
Suzuki K, Min CK, Kim K, Lee JJ, Shibayama H, Ko PS, et al. Carfilzomib, dexamethasone, and daratumumab in Asian patients with relapsed or refractory multiple myeloma: post hoc subgroup analysis of the phase 3 CANDOR trial. Int J Hematol. 2021;114(6):653–63. https://doi.org/10.1007/s12185-021-03204-9.
Iida S, Ishikawa T, Min CK, Kim K, Yeh SP, Usmani SZ, et al. Subcutaneous daratumumab in Asian patients with heavily pretreated multiple myeloma: subgroup analyses of the noninferiority, phase 3 COLUMBA study. Ann Hematol. 2021;100(4):1065–77. https://doi.org/10.1007/s00277-021-04405-2.
Fujisaki T, Ishikawa T, Takamatsu H, Suzuki K, Min CK, Lee JH, et al. Daratumumab plus bortezomib, melphalan, and prednisone in East Asian patients with non-transplant multiple myeloma: subanalysis of the randomized phase 3 ALCYONE trial. Ann Hematol. 2019;98(12):2805–14. https://doi.org/10.1007/s00277-019-03794-9.
Salles G, Gopal AK, Minnema MC, Wakamiya K, Feng H, Schecter JM, et al. Phase 2 study of daratumumab in relapsed/refractory mantle-cell lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19(5):275–84. https://doi.org/10.1016/j.clml.2018.12.013.
Huang H, Zhu J, Yao M, Kim TM, Yoon DH, Cho SG, et al. Daratumumab monotherapy for patients with relapsed or refractory natural killer/T-cell lymphoma, nasal type: an open-label, single-arm, multicenter, phase 2 study. J Hematol Oncol. 2021;14(1):25. https://doi.org/10.1186/s13045-020-01020-y.
Usmani SZ, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): updated outcomes from a randomised, multicentre, open-label, phase 3 study. Lancet Oncol. 2022;23(1):65–76. https://doi.org/10.1016/S1470-2045(21)00579-9.
Kumar SK, Facon T, Usmani SZ, Plesner T, Orlowski RZ, Touzeau C, et al. Updated analysis of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with transplant-ineligible newly diagnosed multiple myeloma (NDMM): the phase 3 Maia Study. Blood. 2020;5:136. https://doi.org/10.1182/blood-2020-134847.
Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395(10218):132–41. https://doi.org/10.1016/S0140-6736(19)32956-3.
Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–45. https://doi.org/10.1182/blood.2020005288.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
Kyeongmin Kim and Mitch A. Phelps have no conflicts of interest that are directly relevant to the content of this review.
Authors’ Contributions
All authors contributed to the literature search, drafting, and editing of the manuscript.
Funding
Support for this work included funding from the US National Cancer Institute, grants U24CA247648 and R01CA273924.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, K., Phelps, M.A. Clinical Pharmacokinetics and Pharmacodynamics of Daratumumab. Clin Pharmacokinet 62, 789–806 (2023). https://doi.org/10.1007/s40262-023-01240-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-023-01240-8