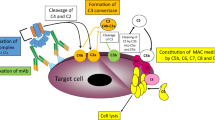
Abstract
The complement system is a crucial part of the innate immune response, providing defense against invading pathogens and cancer cells. Recently, it has become evident that the complement system plays a significant role in anticancer activities, particularly through complement-dependent cytotoxicity (CDC), alongside antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cell-mediated phagocytosis (ADCP). With the discovery of new roles for serum complement molecules in the human immune system, various approaches are being pursued to develop CDC-enhanced antibody therapeutics. In this review, we focus on successful antibody engineering strategies for enhancing CDC, analyzing the lessons learned and the limitations of each approach. Furthermore, we outline potential pathways for the development of antibody therapeutics specifically aimed at enhancing CDC for superior therapeutic efficacy in the future.




Similar content being viewed by others
References
Walport MJ. Complement. N Engl J Med. 2001;344(14):1058–66. https://doi.org/10.1056/NEJM200104053441406.
Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18(1):46–61. https://doi.org/10.1038/nri.2017.106.
Lu Y, Zhao Q, Liao J-Y, Song E, Xia Q, Pan J, et al. Complement signals determine opposite effects of b cells in chemotherapy-induced immunity. Cell. 2020;180(6):1081-97.e24. https://doi.org/10.1016/j.cell.2020.02.015.
Afshar-Kharghan V. The role of the complement system in cancer. J Clin Investig. 2017;127(3):780–9. https://doi.org/10.1172/JCI90962.
Kang TH, Jung ST. Boosting therapeutic potency of antibodies by taming Fc domain functions. Exp Mol Med. 2019;51(11):1–9. https://doi.org/10.1038/s12276-019-0345-9.
Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, et al. Complement activation determines the therapeutic activity of rituximab in vivo 1. J Immunol. 2003;171(3):1581–7. https://doi.org/10.4049/jimmunol.171.3.1581.
Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, van de Winkel JGJ, Parren PWHI, et al. Binding of Submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX1. J Immunol. 2009;183(1):749–58. https://doi.org/10.4049/jimmunol.0900632.
Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6(4):443–6. https://doi.org/10.1038/74704.
Weng WK, Levy R. Expression of complement inhibitors CD46, CD55, and CD59 on tumor cells does not predict clinical outcome after rituximab treatment in follicular non-Hodgkin lymphoma. Blood. 2001;98(5):1352–7. https://doi.org/10.1182/blood.v98.5.1352.
Zent CS, Secreto CR, LaPlant BR, Bone ND, Call TG, Shanafelt TD, et al. Direct and complement dependent cytotoxicity in CLL cells from patients with high-risk early–intermediate stage chronic lymphocytic leukemia (CLL) treated with alemtuzumab and rituximab. Leuk Res. 2008;32(12):1849–56. https://doi.org/10.1016/j.leukres.2008.05.014.
de Weers M, Tai Y-T, van der Veer MS, Bakker JM, Vink T, Jacobs DCH, et al. Daratumumab, a novel therapeutic human cd38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8. https://doi.org/10.4049/jimmunol.1003032.
Freeman CL, Sehn LH. A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol. 2018;182(1):29–45. https://doi.org/10.1111/bjh.15232.
Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–97. https://doi.org/10.1038/ni.1923.
Lee C-H, Romain G, Yan W, Watanabe M, Charab W, Todorova B, et al. IgG Fc domains that bind C1q but not effector Fcγ receptors delineate the importance of complement-mediated effector functions. Nat Immunol. 2017;18(8):889–98. https://doi.org/10.1038/ni.3770.
Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. https://doi.org/10.1038/cr.2009.139.
Kaplon H, Chenoweth A, Crescioli S, Reichert JM. Antibodies to watch in 2022. MAbs. 2022;14(1):2014296. https://doi.org/10.1080/19420862.2021.2014296.
Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164(8):4178–84. https://doi.org/10.4049/jimmunol.164.8.4178.
Idusogie EE, Wong PY, Presta LG, Gazzano-Santoro H, Totpal K, Ultsch M, et al. Engineered antibodies with increased activity to recruit complement. J Immunol. 2001;166(4):2571–5. https://doi.org/10.4049/jimmunol.166.4.2571.
Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2(2):181–9. https://doi.org/10.4161/mabs.2.2.11158.
Ugurlar D, Howes SC, de Kreuk B-J, Koning RI, de Jong RN, Beurskens FJ, et al. Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science. 2018;359(6377):794–7. https://doi.org/10.1126/science.aao4988.
Caaveiro JMM, Kiyoshi M, Tsumoto K. Structural analysis of Fc/FcγR complexes: a blueprint for antibody design. Immunol Rev. 2015;268(1):201–21. https://doi.org/10.1111/imr.12365.
Hughes-Jones NC, Gardner B. Reaction between the isolated globular sub-units of the complement component Clq and IgG-complexes. Mol Immunol. 1979;16(9):697–701. https://doi.org/10.1016/0161-5890(79)90010-5.
Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260–3. https://doi.org/10.1126/science.1248943.
de Jong RN, Beurskens FJ, Verploegen S, Strumane K, van Kampen MD, Voorhorst M, et al. A novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLOS Biol. 2016;14(1): e1002344. https://doi.org/10.1371/journal.pbio.1002344.
Simone CO, JvdH H, Margaret AL, Erika MC, Jillian CT, Clive SZ, et al. CD20 and CD37 antibodies synergize to activate complement by Fc-mediated clustering. Haematologica. 2019;104(9):1841–52. https://doi.org/10.3324/haematol.2018.207266.
Oostindie SC, van der Horst HJ, Kil LP, Strumane K, Overdijk MB, van den Brink EN, et al. DuoHexaBody-CD37®, a novel biparatopic CD37 antibody with enhanced Fc-mediated hexamerization as a potential therapy for B-cell malignancies. Blood Cancer J. 2020;10(3):30. https://doi.org/10.1038/s41408-020-0292-7.
Sopp JM, Peters SJ, Rowley TF, Oldham RJ, James S, Mockridge I, et al. On-target IgG hexamerisation driven by a C-terminal IgM tail-piece fusion variant confers augmented complement activation. Commun Biol. 2021;4(1):1031. https://doi.org/10.1038/s42003-021-02513-3.
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. https://doi.org/10.3389/fimmu.2014.00520.
Norderhaug L, Brekke OH, Bremnes B, Sandin R, Aase A, Michaelsen TE, et al. Chimeric mouse human IgG3 antibodies with an IgG4-like hinge region induce complement-mediated lysis more efficiently than IgG3 with normal hinge. Eur J Immunol. 1991;21(10):2379–84. https://doi.org/10.1002/eji.1830211013.
Natsume A, In M, Takamura H, Nakagawa T, Shimizu Y, Kitajima K, et al. Engineered antibodies of IgG1/IgG3 mixed isotype with enhanced cytotoxic activities. Can Res. 2008;68(10):3863–72. https://doi.org/10.1158/0008-5472.Can-07-6297.
Natsume A, Shimizu-Yokoyama Y, Satoh M, Shitara K, Niwa R. Engineered anti-CD20 antibodies with enhanced complement-activating capacity mediate potent anti-lymphoma activity. Cancer Sci. 2009;100(12):2411–8. https://doi.org/10.1111/j.1349-7006.2009.01327.x.
Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, et al. Decoding the human immunoglobulin g-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front Immunol. 2017;8:877. https://doi.org/10.3389/fimmu.2017.00877.
Peschke B, Keller CW, Weber P, Quast I, Lünemann JD. Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front Immunol. 2017;8:646. https://doi.org/10.3389/fimmu.2017.00646.
van Osch TLJ, Nouta J, Derksen NIL, van Mierlo G, van der Schoot CE, Wuhrer M, et al. Fc galactosylation promotes hexamerization of human IgG1, leading to enhanced classical complement activation. J Immunol. 2021;207(6):1545–54. https://doi.org/10.4049/jimmunol.2100399.
Fishelson Z, Kirschfink M. Complement C5b–9 and cancer: mechanisms of cell damage, cancer counteractions, and approaches for intervention. Front Immunol. 2019;10:2. https://doi.org/10.3389/fimmu.2019.00752.
Zhang R, Liu Q, Liao Q, Zhao Y. CD59: a promising target for tumor immunotherapy. Future Oncol. 2018;14(8):781–91. https://doi.org/10.2217/fon-2017-0498.
Barth MJ, Mavis C, Czuczman MS, Hernandez-Ilizaliturri FJ. Ofatumumab exhibits enhanced in vitro and in vivo activity compared to rituximab in preclinical models of mantle cell lymphoma. Clin Cancer Res. 2015;21(19):4391–7. https://doi.org/10.1158/1078-0432.Ccr-15-0056.
Kesselring R, Thiel A, Pries R, Fichtner-Feigl S, Brunner S, Seidel P, et al. The complement receptors CD46, CD55 and CD59 are regulated by the tumour microenvironment of head and neck cancer to facilitate escape of complement attack. Eur J Cancer. 2014;50(12):2152–61. https://doi.org/10.1016/j.ejca.2014.05.005.
Gelderman KA, Blok VT, Fleuren GJ, Gorter A. The inhibitory effect of CD46, CD55, and CD59 on complement activation after immunotherapeutic treatment of cervical carcinoma cells with monoclonal antibodies or bispecific monoclonal antibodies. Lab Invest. 2002;82(4):483–93. https://doi.org/10.1038/labinvest.3780441.
Gelderman KA, Lam S, Sier CF, Gorter A. Cross-linking tumor cells with effector cells via CD55 with a bispecific mAb induces β-glucan-dependent CR3-dependent cellular cytotoxicity. Eur J Immunol. 2006;36(4):977–84. https://doi.org/10.1002/eji.200535653.
Macor P, Secco E, Mezzaroba N, Zorzet S, Durigutto P, Gaiotto T, et al. Bispecific antibodies targeting tumor-associated antigens and neutralizing complement regulators increase the efficacy of antibody-based immunotherapy in mice. Leukemia. 2015;29(2):406–14. https://doi.org/10.1038/leu.2014.185.
Mamidi S, Höne S, Teufel C, Sellner L, Zenz T, Kirschfink M. Neutralization of membrane complement regulators improves complement-dependent effector functions of therapeutic anticancer antibodies targeting leukemic cells. Oncoimmunology. 2015;4(3): e979688. https://doi.org/10.4161/2162402x.2014.979688.
Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128(7):959–70. https://doi.org/10.1182/blood-2016-03-703439.
Wang Y, Yang YJ, Wang Z, Liao J, Liu M, Zhong XR, et al. CD55 and CD59 expression protects HER2-overexpressing breast cancer cells from trastuzumab-induced complement-dependent cytotoxicity. Oncol Lett. 2017;14(3):2961–9. https://doi.org/10.3892/ol.2017.6555.
Kumar A, Planchais C, Fronzes R, Mouquet H, Reyes N. Binding mechanisms of therapeutic antibodies to human CD20. Science. 2020;369(6505):793–9. https://doi.org/10.1126/science.abb8008.
Wang B, Yang C, Jin X, Du Q, Wu H, Dall’Acqua W, et al. Regulation of antibody-mediated complement-dependent cytotoxicity by modulating the intrinsic affinity and binding valency of IgG for target antigen. MAbs. 2020;12(1):1690959. https://doi.org/10.1080/19420862.2019.1690959.
Wang G, de Jong RN, van den Bremer ET, Beurskens FJ, Labrijn AF, Ugurlar D, et al. Molecular basis of assembly and activation of complement component C1 in complex with immunoglobulin G1 and antigen. Mol Cell. 2016;63(1):135–45. https://doi.org/10.1016/j.molcel.2016.05.016.
Mamidi S, Cinci M, Hasmann M, Fehring V, Kirschfink M. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol. 2013;7(3):580–94. https://doi.org/10.1016/j.molonc.2013.02.011.
Kontermann RE, Wing MG, Winter G. Complement recruitment using bispecific diabodies. Nat Biotechnol. 1997;15(7):629–31. https://doi.org/10.1038/nbt0797-629.
Cruz JW, Damko E, Modi B, Tu N, Meagher K, Voronina V, et al. A novel bispecific antibody platform to direct complement activity for efficient lysis of target cells. Sci Rep. 2019;9(1):12031. https://doi.org/10.1038/s41598-019-48461-1.
Pedersen ML, Pedersen DV, Winkler MBL, Olesen HG, Søgaard OS, Ostergaard L, et al. Nanobody-mediated complement activation to kill HIV-infected cells. EMBO Mol Med. 2023;15(4): e16422. https://doi.org/10.15252/emmm.202216422.
Wirt T, Rosskopf S, Rösner T, Eichholz KM, Kahrs A, Lutz S, et al. An Fc double-engineered CD20 antibody with enhanced ability to trigger complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity. Transfus Med Hemother. 2017;44(5):292–300. https://doi.org/10.1159/000479978.
Gehlert CL, Rahmati P, Boje AS, Winterberg D, Krohn S, Theocharis T, et al. Dual Fc optimization to increase the cytotoxic activity of a CD19-targeting antibody. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.957874.
Jebamani P, Sokalingam S, Sriramulu DK, Jung ST, Lee S-G. Assessment of computational modeling of Fc-Fc receptor binding through protein-protein docking tool. Biotechnol Bioprocess Eng. 2020;25(5):734–41. https://doi.org/10.1007/s12257-020-0050-5.
Jebamani P, Sriramulu DK, Jung ST, Lee S-G. Structural study on the impact of S239D/I332E mutations in the binding of Fc and FcγRIIIa. Biotechnol Bioprocess Eng. 2021;26(6):985–92. https://doi.org/10.1007/s12257-021-0024-2.
Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci. 2006;103(11):4005–10. https://doi.org/10.1073/pnas.0508123103.
Revel M, Daugan MV, Sautés-Fridman C, Fridman WH, Roumenina LT. Complement system: promoter or suppressor of cancer progression? Antibodies. 2020;9(4):57.
Introna M, Golay J. Complement in antibody therapy: friend or foe? Blood. 2009;114(26):5247–8. https://doi.org/10.1182/blood-2009-10-249532.
Wang Y, Zhang H, He YW. The complement receptors C3aR and C5aR are a new class of immune checkpoint receptor in cancer immunotherapy. Front Immunol. 2019;10:1574. https://doi.org/10.3389/fimmu.2019.01574.
Kaplon H, Crescioli S, Chenoweth A, Visweswaraiah J, Reichert JM. Antibodies to watch in 2023. MAbs. 2023;15(1):2153410. https://doi.org/10.1080/19420862.2022.2153410.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by the Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN21C0103, Republic of Korea). We are also grateful for support from grants from the Basic Science Research Program (RS-2023-00245059 and 2022R1A4A2000827) through the National Research Foundation of Korea funded by the Ministry of Science and ICT.
Conflicts of interest
Wonju Lee, Sang Min Lee, and Sang Taek Jung declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions.
All authors reviewed and commented on the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, W., Lee, S.M. & Jung, S.T. Unlocking the Power of Complement-Dependent Cytotoxicity: Engineering Strategies for the Development of Potent Therapeutic Antibodies for Cancer Treatments. BioDrugs 37, 637–648 (2023). https://doi.org/10.1007/s40259-023-00618-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-023-00618-1