Abstract
Introduction
Gram-negative resistance is a well-acknowledged public health threat. Surveillance data can be used to monitor resistance trends and identify strategies to mitigate their threat. The objective of this study was to assess antibiotic resistance trends in Gram-negative bacteria.
Methods
The first cultures of Pseudomonas aeruginosa, Citrobacter, Escherichia coli, Enterobacter, Klebsiella, Morganella morganii, Proteus mirabilis, and Serratia marcescens per hospitalized patient per month collected from 125 Veterans Affairs Medical Centers (VAMCs) between 2011 to 2020 were included. Time trends of resistance phenotypes (carbapenem, fluoroquinolone, extended-spectrum cephalosporin, multi-drug, and difficult-to-treat) were analyzed with Joinpoint regression to estimate average annual percent changes (AAPC) with 95% confidence intervals and p values. A 2020 antibiogram of reported antibiotic percent susceptibilities was also created to evaluate resistance rates at the beginning of the COVID-19 pandemic.
Results
Among 40 antimicrobial resistance phenotype trends assessed in 494,593 Gram-negative isolates, there were no noted increases; significant decreases were observed in 87.5% (n = 35), including in all P. aeruginosa, Citrobacter, Klebsiella, M. morganii, and S. marcescens phenotypes (p < 0.05). The largest decreases were seen in carbapenem-resistant phenotypes of P. mirabilis, Klebsiella, and M. morganii (AAPCs: − 22.9%, − 20.7%, and − 20.6%, respectively). In 2020, percent susceptibility was over 80% for all organisms tested against aminoglycosides, cefepime, ertapenem, meropenem, ceftazidime-avibactam, ceftolozane-tazobactam, and meropenem-vaborbactam.
Conclusion
We observed significant decreases in antibiotic resistance for P. aeruginosa and Enterobacterales over the past decade. According to the 2020 antibiogram, in vitro antimicrobial activity was observed for most treatment options. These results may be related to the robust infection control and antimicrobial stewardship programs instituted nationally among VAMCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Antibiotic-resistant Gram-negative bacilli, including Enterobacterales and Pseudomonas aeruginosa, have been identified as public health threats due to their ability to demonstrate resistance to all available antibiotics and cause nosocomial outbreaks. |
The objective of this study was to assess antibiotic resistance trends in Gram-negative bacteria at Veterans Affairs Medical Centers from 2011 to 2020. |
What was learned from the study? |
Among 40 antimicrobial resistance phenotype trends assessed in 494,593 Gram-negative isolates, there were no noted increases, and significant decreases were observed in 87.5% of phenotypes, indicating favorable trends in Gram-negative antibiotic resistance at Veterans Affairs Medical Center from 2011 to 2020. |
Factors associated with these decreases, such as antibiotic utilization, molecular characteristics of antimicrobial resistance, and the effects of infection control and antimicrobial stewardship interventions, will need to be examined to identify effective implementation strategies to prevent future increases in antimicrobial resistance locally, regionally, and nationally. |
Introduction
Antimicrobial resistance (AR) is a significant global health threat, leading to approximately 2.8 million infections and 35,000 deaths annually in the United States (US) [1]. Gram-negative bacilli, including Enterobacterales and Pseudomonas aeruginosa, can demonstrate resistance to all available antibiotics through various resistance mechanisms [2]. Further, they can cause nosocomial outbreaks, which has led to the global emergence and dissemination of Gram-negative bacterial strains that harbor high-level resistance due to extended-spectrum beta-lactamases such as carbapenemases [3]. The spread of these AR phenotypes can limit the utility of traditional first-line treatment options and negatively affect vulnerable populations and healthcare systems [4,5,6].
The control and treatment of resistant Gram-negative pathogens are the focus of public health initiatives from the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC), which include infection control and antimicrobial stewardship programs (IC/ASPs) [1, 7, 8]. While various regulatory bodies have mandated IC/ASPs, the effects of their implementation need to be assessed to determine successes and shortcomings and where to target future initiatives in these areas [1, 8,9,10,11]. To support these efforts, surveillance systems for monitoring AR have been developed, including facility-level reporting with the CDC’s National Healthcare Safety Network (NHSN) and country-level reporting through WHO’s Global Antimicrobial Resistance Surveillance System [1, 8, 12, 13].
Data on overall US resistance trends are largely derived from CDC modeling estimates, with smaller surveillance studies at times reporting differing trends [1, 14]. Since 2012, decreases in carbapenem-resistant Acinetobacter have been observed; however, there have been conflicting reports on trends in multi-drug-resistant P. aeruginosa [1, 15,16,17]. For Enterobacterales, extended-spectrum cephalosporin-resistant infections have increased in both community and healthcare settings, with varied trends reported for carbapenem-resistant infections [1, 16,17,18]. Given these differences, existing data should be confirmed by alternate studies. Furthermore, CDC modeling estimates do not include federal hospitals, which notably excludes the US’s largest integrated healthcare system, the Veterans Health Administration (VHA).
Additional surveillance data are needed to elucidate US AR trends, discern the effects of existing IC/ASPs, and inform practical strategies to minimize the future impact of AR. Therefore, the purpose of this study was to assess Gram-negative resistance (GNR) trends over the previous decade in patients hospitalized at Veterans Affairs Medical Centers (VAMCs).
Methods
Clinical cultures (first culture per patient per month) of P. aeruginosa and select Enterobacterales (Citrobacter species, Escherichia coli, Enterobacter species, Klebsiella species, Morganella morganii, Proteus mirabilis, and Serratia marcescens) with reported antimicrobial susceptibilities collected from patients hospitalized at 125 VAMCs from 2011 to 2020 were included. The site of collection was categorized as blood, respiratory, urine, skin and tissue, or other. When multiple cultures were collected on the same day from the same patient, the most sterile site was selected for inclusion.
Resistance was defined as not susceptible (intermediate or resistant) to that antibiotic or antibiotic class. The most recent Clinical Laboratory Standards Institute (CLSI) breakpoints were utilized to determine non-susceptibility when numeric minimum inhibitory concentration (MIC) data were available [19,20,21]. When MIC values were not available, the reported textual interpretations were utilized (i.e., resistant, intermediate, or susceptible). In cases of duplicate cultures (same patient, culture site, organism, and day) but conflicting antimicrobial susceptibility results, the most resistant result was included (i.e., resistant > intermediate > susceptible).
The following AR phenotypes were assessed: fluoroquinolone resistance, extended-spectrum cephalosporin resistance (ESCR), carbapenem resistance, multi-drug resistance (MDR), and difficult-to-treat resistance (DTR), as defined in Table 1. Other than DTR, a newer definition characterized by resistance to all first-line recommended antibiotics, definitions were based on 2021 NHSN definitions or as otherwise specified [6, 22,23,24]. Time trends were assessed with Joinpoint regression to estimate average annual percent changes (AAPCs) of AR phenotypes with 95% confidence intervals (CIs) and p values. Joinpoint Trend Analysis Software (National Cancer Institute, version 4.9.1.0) calculates the annual percent change (APC) when study data are similar over the study years (i.e., a single segment), while additional APCs are calculated (i.e., additional segments) when changes occur in subsequent study years. The average of all the APCs, the AAPC, describes the trends over the entire study period and tests for statistical significance at p < 0.05.
A 2020 antibiogram was developed based on monthly reported antibiotic susceptibilities for included isolates to determine if individual drug–organism combinations retained a percent susceptibility of over 80%. If fewer than 30 isolates were reported for an antibiotic against a particular organism, those numbers were excluded due to concerns about the statistical validity of the reported percent susceptibility [25]. Drug–species combinations with intrinsic resistance per the CLSI were also excluded [19].
This study was approved by the Veterans Affairs Central Institutional Review Board (reference no. 18-33) with a waiver of informed consent. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Results
Pathogens and Culture Sites
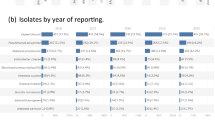
A total of 494,593 clinical Gram-negative isolates from 257,585 patients hospitalized at 125 VAMCs from 2011 to 2020 were included. The most common organism was E. coli (32.5%), followed by Klebsiella (20.0%), P. aeruginosa (19.0%), P. mirabilis (11.5%), Enterobacter (7.8%), Citrobacter (3.7%), S. marcescens (2.9%), and M. morganii (2.6%). The most common site of collection for each organism was urine (Fig. 1).
Distribution of organisms (n = 494,593) by culture site. Included organisms are ordered on the x axis in the figure by the most prevalent, and each organism was stratified by percentage into the following collection sites on the y axis: urine, respiratory, skin and tissue, blood, and other. The “other” category included, but was not limited to, the following collection sites: bone and joint, catheter, cerebrospinal fluid, intra-abdominal
Antimicrobial Resistance Trends
From 2011 to 2020, resistance in all phenotypes decreased or remained stable, with no noted increases. The largest decreases were observed in carbapenem-resistant P. mirabilis, Klebsiella, M. morganii, and E. coli, and in DTR S. marcescens and M. morganii (AAPCs: − 22.9%, − 20.7%, − 20.6%, − 17.5%, − 19.4%, and − 17.9%, respectively).
For P. aeruginosa (Fig. 2), fluoroquinolone resistance was the most common phenotype observed, which decreased 6.6% annually (95% CI − 7.4 to − 5.7%; 36% in 2011, 19% in 2020). ESCR decreased 3.9% annually (95% CI − 4.9 to − 3%; 23% in 2011, 14.5% in 2020), carbapenem resistance decreased 4.6% annually (95% CI − 6.1 to − 3.1%; 23.4% in 2011, 15.1% in 2020), MDR decreased 7.4% annually (95% CI − 9.4 to − 5.2%, 19.3% in 2011, 8.9% in 2020), and DTR decreased 9% annually (95% CI − 13.1 to − 4.6%; 7.7% in 2011, 3.1% in 2020).
Trends in antimicrobial resistance phenotypes for Pseudomonas aeruginosa, Veterans Affairs Medical Centers, 2011–2020. Time trends indicated by average annual percent changes (AAPCs), 95% confidence intervals (CIs), and p values for the following antimicrobial resistance phenotypes: multi-drug resistance (MDR), difficult-to-treat resistance (DTR), extended-spectrum cephalosporin resistance (ESCR), carbapenem resistance (CR), and fluoroquinolone resistance (FR). Percent resistance on the y axis, maximum scale value of 40%
For Citrobacter (Fig. 3a), the most common phenotype observed was ESCR, which decreased 4% annually (95% CI − 5.2 to − 2.7%; 25% in 2011, 18.4% in 2020). Fluoroquinolone resistance decreased 8.9% annually (95% CI − 11 to − 6.8%; 15.8% in 2011, 6.7% in 2020), and carbapenem resistance decreased 9.9% annually (95% CI − 15.5 to − 3.9%; 6.7% in 2011, 2.3% in 2020). For MDR, rates decreased 9% annually (95% CI − 12.7 to − 5.1%; 6% in 2011, 2.4% in 2020). Rates of DTR were low and decreased 12% annually (95% CI − 18 to − 5.5%; 1% in 2011, 0.3% in 2020).
Trends in antimicrobial resistance phenotypes for a Citrobacter species, b Enterobacter species, c Klebsiella species, d Serratia marcescens, Veterans Affairs Medical Centers, 2011–2020. Time trends indicated by average annual percent changes (AAPCs), 95% confidence intervals (CIs), and p values for the following antimicrobial resistance phenotypes: multi-drug resistance (MDR), difficult-to-treat resistance (DTR), extended-spectrum cephalosporin resistance (ESCR), carbapenem resistance (CR), and fluoroquinolone resistance (FR). Percent resistance on the y axis, maximum scale value of 40% for all figures
For E. coli (Fig. 4a), fluoroquinolone resistance was the most common phenotype observed, which decreased 2.6% annually (95% CI − 3.3 to − 1.9%; 41.9% in 2011, 32.7% in 2020); carbapenem resistance decreased by 17.5% annually (95% CI − 27.4 to − 6.3%; 4.9% in 2011, 0.4% in 2020). Over the same period, stable rates of ESCR (p = 0.6; 17.3% in 2020), MDR (p = 0.1; 6.9% in 2020), and DTR (p = 0.4; 1.8% in 2020) were observed.
Trends in antimicrobial resistance phenotypes for a Escherichia coli, b Morganella morganii, c Proteus mirabilis, Veterans Affairs Medical Centers, 2011–2020. Time trends indicated by average annual percent changes (AAPCs), 95% confidence intervals (CIs), and p values for the following antimicrobial resistance phenotypes: multi-drug resistance (MDR), difficult-to-treat resistance (DTR), extended-spectrum cephalosporin resistance (ESCR), carbapenem resistance (CR), and fluoroquinolone resistance (FR). Percent resistance on the y axis, maximum scale value of 55% for all figures
For Enterobacter (Fig. 3b), carbapenem resistance was the most common phenotype observed, which decreased 3.2% annually (95% CI − 4.8 to − 1.6%; 13.4% in 2011, 10.5% in 2020). Fluoroquinolone resistance decreased 9.5% annually (95% CI − 11.1 to − 7.8%; 12.4% in 2011, 4.6% in 2020), MDR decreased 6.6% annually (95% CI − 9.1 to − 4%; 6% in 2011, 3% in 2020), and DTR decreased 16.3% annually (95% CI − 21.6 to − 10.5%; 2.1% in 2011, 0.6% in 2020). For ESCR, including third-generation cephalosporins (i.e., ceftriaxone, cefotaxime, and ceftazidime), resistance decreased 2.4% annually (95% CI − 3.3 to − 1.6%; 32.2% in 2011, 27.2% in 2020; data not shown in Figure). For an ESCR definition that only included cefepime, trends were stable and remained low at 6.2% in 2020 (p = 0.2).
For Klebsiella (Fig. 3c), the most common phenotype observed was ESCR, which decreased 5.4% annually (95% CI − 6.7 to − 4.1%; 24.2% in 2011, 14.6% in 2020). Fluoroquinolone resistance decreased 5.3% annually (95% CI − 7 to − 3.6%; 18% in 2011, 11.7% in 2020), carbapenem resistance decreased 20.7% annually (95% CI − 25 to − 16.1%; 10.6% in 2011, 1.5% in 2020), MDR decreased 8.4% annually (95% CI − 10.5 to − 6.3%; 13.7% in 2011, 7.4% in 2020), and DTR decreased 16.2% annually (95% CI − 28 to − 2.3%; 4.3% in 2011, 0.8% in 2020).
For Morganella morganii (Fig. 4b), fluoroquinolone resistance was the most common phenotype observed, which decreased 5.7% annually (95% CI − 6.9 to − 4.4%; 47.1% in 2011, 27.5% in 2020). Carbapenem resistance decreased 20.6% annually (95% CI − 28.7 to − 11.6%; 5% in 2011, 1% in 2020), ESCR decreased 5.3% annually (95% CI − 6.5 to − 4%; 34.9% in 2011, 22.7% in 2020), MDR decreased 9.3% annually (95% CI − 11 to − 7.6%, 16% in 2011, 7% in 2020), and DTR decreased 17.9% annually (95% CI − 23 to − 12.4%; 5% in 2011, 0.7% in 2020).
For Proteus mirabilis (Fig. 4c), fluoroquinolone resistance was the most common phenotype observed, and decreased 4.2% annually (95% CI − 5.6 to − 2.8%; 49.7% in 2011, 33.9% in 2020). Carbapenem resistance decreased 22.9% annually (95% CI − 31.6 to − 13.1%; 7% in 2011, 1% in 2020), ESCR decreased 8.4% annually (95% CI − 10.8 to − 6%; 19% in 2011, 7.9% in 2020), and MDR decreased 6.7% annually (95% CI − 9.3 to − 4%, 6% in 2011, 3% in 2020). Rates of DTR remained low and stable at 1.7% in 2020 (p = 0.7).
For Serratia marcescens (Fig. 3d), the most common phenotype observed was ESCR, which decreased 5.3% annually (95% CI − 8.4 to − 2.2%; 23.1% in 2011, 14.1% in 2020). Fluoroquinolone resistance decreased 9.9% annually (95% CI − 12 to − 7.8%; 12.2% in 2011, 4.6% in 2020), carbapenem resistance decreased 12.3% annually (95% CI − 16.2 to − 8.1%; 14.5% in 2011, 4.2% in 2020), MDR decreased 12.2% annually (95% CI − 14.4 to − 9.9%; 6.7% in 2011, 2.5% in 2020), and DTR decreased 19.4% annually (95% CI − 32.3 to − 4.1%; 0.6% in 2011, 0% in 2020).
Antimicrobial Resistance Phenotype Prevalence in 2020
In 2020, ESCR was seen most in M. morganii (22.7%), Citrobacter (18.4%), and E. coli (17.3%), and least in P. mirabilis (7.9%) and Enterobacter (6.2%). Fluoroquinolone resistance was seen most in P. mirabilis (33.9%), E. coli (32.7%), and M. morganii (27.5%), and least in Enterobacter and S. marcescens (both 4.6%). P. aeruginosa had the highest rates of carbapenem resistance (15.1%), MDR (8.9%), and DTR (3.1%). For Enterobacterales, carbapenem resistance was seen most in Enterobacter (10.5%), S. marcescens (4.2%), and Citrobacter (2.3%), and least in E. coli (0.4%). MDR was seen most in Klebsiella (7.4%), M. morganii (7%), and E. coli (6.9%), and least in S. marcescens (2.5%) and Citrobacter (2.4%), and DTR was seen most in E. coli (1.8%), P. mirabilis (1.7%), and Klebsiella (0.8%), and least in Citrobacter (0.3%) and S. marcescens (0%).
2020 Antibiogram
Table 2 provides a 2020 antibiogram for the included isolates. Most beta-lactam antibiotics retained PS80 [26]. Ampicillin-sulbactam, the narrowest-spectrum beta-lactam included in the antibiogram, had PS80 only for P. mirabilis. Aztreonam, ertapenem, and meropenem-vaborbactam retained PS80 for all Enterobacterales. Ceftriaxone, ceftazidime, and piperacillin-tazobactam had PS80 for all organisms aside from Enterobacter. Cefepime, ceftazidime-avibactam, and ceftolozane-tazobactam retained PS80 for all organisms. Imipenem retained PS80 for all organisms with the exception of M. morganii and P. mirabilis.
Among non-beta-lactam antibiotics, aminoglycosides retained PS80 for all organisms. Trimethoprim-sulfamethoxazole retained PS80 for S. marcescens, Citrobacter, Enterobacter, and Klebsiella. Tetracycline retained PS80 for Citrobacter and Enterobacter. Nitrofurantoin retained PS80 for Citrobacter and E. coli. Ciprofloxacin retained PS80 for Citrobacter, Enterobacter, Klebsiella, S. marcescens, and P. aeruginosa.
Discussion
Over the previous decade, among almost 500,000 Gram-negative clinical cultures from patients hospitalized at VAMCs nationally, significant decreases were observed in 87.5% of AR phenotypes assessed (n = 35/40), including all P. aeruginosa, Citrobacter, Klebsiella, M. morganii, and S. marcescens phenotypes. No increasing trends were noted. Carbapenem resistance in all organisms decreased significantly, with many Enterobacterales seeing their highest declines in this phenotype.
Rates of carbapenem and fluoroquinolone-resistant P. aeruginosa and ESCR P. aeruginosa, Citrobacter, Klebsiella, and S. marcescens decreased below the 20% threshold, indicating greater availability of empiric antibiotic options for these organisms through the study period [26]. The largest decreases were seen in carbapenem-resistant Klebsiella, M. morganii, E. coli, and P. mirabilis, and in DTR M. morganii and S. marcescens. However, as 2011 AR rates were already less than 20%, the observed decreases do not expand but rather preserve empiric treatment options for these organisms. While significant decreases were also seen in fluoroquinolone-resistant E. coli, M. morganii, and P. mirabilis, and in ESCR M. morganii, resistance rates remained over 20% in 2020, signaling that fluoroquinolones may still not be an appropriate empiric treatment for these organisms. Also, considering the boxed warnings regarding their safety profile and Gram-negative bacteria’s ability to acquire multiple resistance mechanisms that may be precipitated by prior fluoroquinolone exposure, continuing to avoid fluoroquinolone use is a prudent treatment approach if other empiric options are available [27, 28].
In 2020, MDR rates were less than 10% and DTR rates were less than 1% other than for P. aeruginosa (3.1%), E. coli (1.8%), and P. mirabilis (1.7%). These low rates agree with antibiotic susceptibilities from the 2020 antibiogram, which demonstrates PS80 to numerous antibiotics, including aminoglycosides, cefepime, ertapenem, meropenem, ceftazidime-avibactam, ceftolozane-tazobactam, and meropenem-vaborbactam for all organisms. The resistance observed in our study agrees with the known intrinsic imipenem resistance of P. mirabilis and M. morganii and the possible induction of ampC leading to ESCR in certain Enterobacter species with third-generation cephalosporin or piperacillin-tazobactam exposure [9, 29].
To our knowledge, this is the first study to evaluate multiple AR trends, including the newer concept of DTR, for common Gram-negative organisms within the largest integrated healthcare system in the US. The encouraging results seen may have been influenced by the VHA’s national directives for IC/ASPs [10, 30]. The VHA’s 2007 Methicillin-Resistant Staphylococcus aureus (MRSA) Prevention Initiative was associated with declines in hospital-onset Gram-negative bacteremias and other AR threats, including MRSA, vancomycin-resistant Enterococcus, and Clostridioides difficile [18, 30,31,32]. The implementation of the VHA Antimicrobial Stewardship Taskforce in January 2014 mandated all VAMCs to develop ASPs and report to the NHSN Antibiotic Use module [10, 33]. By 2015, 92% of VAMCs had developed a written AS policy, as compared to the 68% of acute care hospitals reporting to NHSN which met the CDC Core Element of Leadership Commitment [11, 33, 34]. A VA study assessing inpatient antibiotic utilization found decreases in antibiotic days of therapy (DOT), including broad-spectrum antibiotics, from 2015 to 2019, which agrees with the decreases in AR seen in this study [35].
While direct comparisons of these results to previous reports are limited by methodological differences in the included organisms and resistance definitions, we were able to roughly compare reported CDC trends to our data using their pathogen-specific phenotype definitions. From 2012 to 2017, whereas CDC found stable rates of carbapenem-resistant E. coli, Klebsiella, and Enterobacter, we observed a 72% decrease. For ESCR E. coli and Klebsiella, CDC observed a 50% increase, compared to the 24% decrease we observed. For MDR P. aeruginosa, CDC saw a 29% decrease, compared to the 35% decrease we observed [1, 22, 23]. While it should be reiterated that these comparisons are not direct, as CDC relied on modeling estimates that were not used in this study, the overall estimated trend differences suggest favorable resistance profiles at VAMCs. Earlier surveillance reports in the VHA also suggest an attenuation of AR in VAMCs, as AR decreases were seen in Acinetobacter from 2010 to 2018, there were stable rates of ESCR Klebsiella pneumoniae bloodstream infections from 2007 to 2013, and decreases in carbapenem-resistant K. pneumoniae were observed from 2006 to 2015 [15, 36, 37].
There were some notable limitations of our study. Some of our phenotype definitions differ from those used in other reports. For example, CDC’s carbapenem-resistant definition only includes isolates that tested resistant to a carbapenem. We opted to include both resistant and intermediate interpretations to stay consistent with other definitions used in this study, and thus may have classified more isolates as carbapenem-resistant compared to CDC [1, 22, 23]. Other notable differences in phenotype definitions include the grouping of multiple Enterobacterales species under specific phenotypes in previous studies (i.e., combining E. coli, K. pneumoniae, K. oxytoca, and P. mirabilis under the “extended-spectrum beta-lactamase” phenotype) and our study categorizing Klebsiella, Enterobacter, and Citrobacter by genera due to lower numbers across the various species, making us unable to assess AR trends in specific species associated with inducible ampC expression of beta-lactamase resistance (i.e., Enterobacter cloacae, Klebsiella aerogenes, and Citrobacter freundii) or according to taxonomic reclassifications that occurred during the study period (i.e., Enterobacter aerogenes changing to Klebsiella aerogenes) [17, 18, 29]. Thus, caution is warranted when interpreting AR in these organisms stratified at the genus level. Since testing methods are not standardized across VHA laboratories, only half of included culture results contain MICs, and facility-level testing methods were not available in the data source, the variation in MIC interpretations could be due to differiences in methods utilized across laboratories or delays in updating to recommended MIC breakpoints, which we were unable to account for [25, 38]. Further, since we relied on reported susceptibilities, suppressed susceptibilities from cascade reporting by individual facilities were not available, which would have impacted our reported resistance rates (i.e., missing data in the numerator and denominator). Additionally, antibiotic susceptibility interpretations are limited to phenotypic detection of AR, which is not confirmatory of specific AR genotypes in the absence of molecular testing such as polymerase chain reaction or whole-genome sequencing. Thus, we were unable to characterize true rates of extended-spectrum beta-lactamases seen in ESCR or carbapenemases in carbapenem-resistant isolates. We were also unable to determine if clinical cultures represented true infection or colonization. While the included isolates were defined as clinical cultures, this does not preclude the possibility that some of the included isolates were collected for surveillance purposes (e.g., in relation to local outbreaks). The 2020 antibiogram included isolates per patient per month rather than per year, so some duplicate isolates which overstate the reported resistance may have been included [25]. Some antibiotics included in the antibiogram were also tested in smaller numbers relative to other antibiotics, which may affect their statistical validity. While these data provide a comprehensive overview of GNR in hospitalized veterans (a population historically known to be predominantly white and male, although we did not collect demographic data to confirm this with our data), caution is warranted when comparing results of this study to other surveillance reports or populations given these limitations, and these results do not stand alone to inform empiric treatment options. Further, as previous work has demonstrated geographical variation in Gram-negative AR, the use of local AR rates to inform IC/ASP interventions is preferred [34, 39].
Nevertheless, given the breadth of this study, our results provide additional evidence of decreasing trends in important AR phenotypes in VAMCs, and they can be used to benchmark GNR rates in VAMCs for IC/ASP-related initiatives. While these positive results may indicate that the VHA has been successful against the threat of AR, this threat is not static, as evidenced by reported effects of the coronavirus disease 2019 (COVID-19) pandemic: a recent CDC report highlighted delays in surveillance reporting, high levels of antibiotic use for hospitalized COVID-19 patients in 2020 (~ 80%), and increases in many hospital-onset AR infections from 2019 to 2020, including for the GNR included in our study [40]. The aforementioned VA study noting decreases in inpatient antibiotic DOT from 2015 to 2019 found increasing DOT in 2020, with a corresponding trend for decreasing narrow-spectrum DOT from 2015 to 2019 that reversed in 2020; these effects persisted when facilities heavily impacted by COVID-19 were excluded [35]. While increases in healthcare-associated infections have also been reported in VAMCs, we did not identify any meaningful differences in AR when comparing rates in 2019 to those in 2020 [24, 41].
These recent reports underscore the importance of continuing to collect robust resistance data to improve antibiotic use in clinical settings, where knowledge of local epidemiology can be used in conjunction with rapid diagnostic testing to streamline appropriate narrow-spectrum empiric treatments. Increased utilization of narrow-spectrum antibiotics may minimize the collateral damage from broad-spectrum antibiotic use, such as secondary AR or C. difficile infections, and help provide a framework to attenuate AR through quality improvement initiatives. Since evaluating overall AR trends was the objective of this study, assessing changes in AR between years was beyond the scope of this study, but this is an important future direction to determine etiologies of AR changes.
Conclusion
In conclusion, the low DTR rates combined with overall decreases in AR seen in this study indicate favorable trends in GNR at VAMCs from 2011 to 2020. Factors associated with these decreases, such as antibiotic utilization, molecular characteristics of AR, and the effects of IC/AS interventions, will need to be examined to identify effective implementation strategies in settings experiencing increasing AR and to prevent future increases in AR locally, regionally, and nationally.
References
CDC. Antibiotic resistance threats in the United States, 2019. Atlanta: US Centers for Disease Control and Prevention; 2019.
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81.
Eichenberger EM, Thaden JT. Epidemiology and mechanisms of resistance of extensively drug resistant gram-negative bacteria. Antibiotics (Basel). 2019;8(2):37.
Walters MS, Witwer M, Lee YK, et al. Notes from the field: carbapenemase-producing carbapenem-resistant Enterobacteriaceae from less common Enterobacteriaceae genera—United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2018;67(23):668–9.
Mammina C, Palma DM, Bonura C, et al. Outbreak of infection with Klebsiella pneumoniae sequence type 258 producing Klebsiella pneumoniae carbapenemase 3 in an intensive care unit in Italy. J Clin Microbiol. 2010;48(4):1506–7.
Kadri SS, Adjemian J, Lai YL, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67(12):1803–14.
WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: World Health Organization; 2017.
WHO. WHO policy guidance on integrated antimicrobial stewardship activities. Geneva: World Health Organization; 2021.
CDC. Facility guidance for control of carbapenem-resistant Enterobacteriaceae. In: CRE Toolkit. Atlanta: US Centers for Disease Control and Prevention; 2015. https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed 10 Jan 2022.
VHA. Antimicrobial stewardship programs (ASP) (VHA Directive 1031). Washington, DC: Department of Veterans Affairs; 2014. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8195. Updated January 2014. Accessed 10 Jan 2022.
CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: US Centers for Disease Control and Prevention; 2019. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. Accessed 10 Jan 2022.
Johnson AP. Surveillance of antibiotic resistance. Philos Trans R Soc Lond B Biol Sci. 2015;370(1670):20140080.
WHO. Global antimicrobial resistance and use surveillance system (GLASS) report: 2021. Geneva: World Health Organization; 2021.
Jernigan JA, Hatfield KM, Wolford H, et al. Multidrug-resistant bacterial infections in US hospitalized patients, 2012–2017. N Engl J Med. 2020;382(14):1309–19.
Appaneal HJ, O’Neill E, Lopes VV, LaPlante KL, Caffrey AR. National trends in hospital, long-term care and outpatient Acinetobacter baumannii resistance rates. J Med Microbiol. 2021;70(12):001473.
Shortridge D, Carvalhaes CG, Streit JM, Flamm RK. Susceptibility trends of ceftolozane/tazobactam and comparators when tested against US gram-negative bacterial surveillance isolates (2012–2018). Diagn Microbiol Infect Dis. 2021;100(1):115302.
Gupta V, Ye G, Olesky M, Lawrence K, Murray J, Yu K. Trends in resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States: 2013–2017. BMC Infect Dis. 2019;19(1):742.
Dunne MW, Aronin SI, Yu KC, Watts JA, Gupta V. A multicenter analysis of trends in resistance in urinary Enterobacterales isolates from ambulatory patients in the United States: 2011–2020. BMC Infect Dis. 2022;22(1):194.
CLSI. Performance standards for antimicrobial susceptibility testing: twentieth informational supplement M100–S20. Wayne: Clinical and Laboratory Standards Institute; 2020.
Appaneal HJ, Caffrey AR, Jiang L, Dosa D, Mermel LA, LaPlante KL. Antibiotic resistance rates for Pseudomonas aeruginosa clinical respiratory and bloodstream isolates among the Veterans Affairs Healthcare System from 2009 to 2013. Diagn Microbiol Infect Dis. 2018;90(4):311–5.
Morrill HJ, Morton JB, Caffrey AR, et al. Antimicrobial resistance of Escherichia coli urinary isolates in the Veterans Affairs Health Care System. Antimicrob Agents Chemother. 2017;61(5):e02236-16.
CDC. Antimicrobial-resistant phenotype definitions. Atlanta: US Centers for Disease Control and Prevention; 2022.
CDC. Antibiotic resistance & patient safety portal antibiotic resistance HAI data—phenotype analytical definitions. Atlanta: US Centers for Disease Control and Prevention; 2021.
Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of extended-spectrum beta-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72(7):1109–16.
CLSI. Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline, 4th edn: M39–A4. Malvern: Clinical Laboratory Standards Institute; 2014.
Hughes M-SA, Dosa DM, Caffrey AR, et al. Antibiograms cannot be used interchangeably between acute care medical centers and affiliated nursing homes. J Am Med Dir Assoc. 2020;21(1):72–7.
Jacoby GA. Mechanisms of resistance to quinolones. Clin Infect Dis. 2005;41(Suppl 2):S120-126.
FDA. Drug safety communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. Press release. Silver Spring: US Food and Drug Administration; 2016.
Tamma PD AS, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America antimicrobial-resistant treatment guidance: gram-negative bacterial infections. Arlington: Infectious Diseases Society of America; 2021. https://www.idsociety.org/practice-guideline/amr-guidance-2.0/. Accessed 10 Dec 2021.
VHA. Management of infectious diseases and infection prevention and control programs (VHA Directive 1131). Washington, DC: Department of Veterans Affairs; 2021.
Nelson RE, Goto M, Samore MH, et al. Expanding an economic evaluation of the Veterans Affairs (VA) Methicillin-resistant Staphylococcus aureus (MRSA) prevention initiative to include prevention of infections from other pathogens. Clin Infect Dis. 2021;72(Suppl 1):S50-s58.
Goto M, O’Shea AMJ, Livorsi DJ, et al. The effect of a nationwide infection control program expansion on hospital-onset gram-negative rod bacteremia in 130 Veterans Health Administration Medical Centers: an interrupted time-series analysis. Clin Infect Dis. 2016;63(5):642–50.
Kelly AA, Jones MM, Echevarria KL, et al. A report of the efforts of the Veterans Health Administration National Antimicrobial Stewardship Initiative. Infect Control Hosp Epidemiol. 2017;38(5):513–20.
CDC. Antibiotic resistance & patient safety portal—hospital antibiotic stewardship. Atlanta: US Centers for Disease Control and Prevention; 2022. https://arpsp.cdc.gov/profile/stewardship. Accessed 10 Jan 2022.
Dieringer TD, Furukawa D, Graber CJ, et al. Inpatient antibiotic utilization in the Veterans’ Health Administration during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2021;42(6):751–3.
Gentry CA, Williams RJ 2nd. Trends in susceptibility rates and extended-spectrum beta-lactamase production of Klebsiella pneumoniae in bloodstream infections across the United States Veterans Affairs Healthcare System. Microb Drug Resist. 2015;21(6):590–9.
Wilson BM, El Chakhtoura NG, Patel S, et al. Carbapenem-resistant Enterobacter cloacae in patients from the US Veterans Health Administration, 2006–2015. Emerg Infect Dis. 2017;23(5):878–80.
Humphries RM, Abbott AN, Hindler JA. Understanding and addressing CLSI breakpoint revisions: a primer for clinical laboratories. J Clin Microbiol. 2019;57(6):00203–19.
Aronin SI, Gupta V, Dunne MW, Watts JA, Yu KC. Regional differences in antibiotic-resistant Enterobacterales urine isolates in the United States: 2018–2020. Int J Infect Dis. 2022;119:142–5.
CDC. 2022 special report: COVID-19 US impact on antimicrobial resistance. Atlanta: US Centers for Disease Control and Prevention; 2022.
Evans ME, Simbartl LA, Kralovic SM, et al. Healthcare-associated infections in Veterans Affairs acute-care and long-term healthcare facilities during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2022;44:1–7.
Acknowledgements
The views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by J. Xin Liao, Haley J. Appaneal, Vrishali Lopes, and Aisling R. Caffrey. The first draft of the manuscript was written by J. Xin Liao, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentation
This work was presented, in part, as an oral presentation on the online platform for IDWeek 2021.
Disclosures
Kerry L. LaPlante receives research funding from Merck, AbbVie, Gilead, and Pfizer Pharmaceuticals and has been a speaker/advisor for Ferring Pharmaceuticals, Melinta Therapeutics, AbbVie, and Seres. Aisling R. Caffrey has received research funding from AbbVie, Gilead, Merck, and Shionogi, and has been a speaker/advisor for Merck. J. Xin Liao, Haley J. Appaneal, Anupama Menon, and Vrishali Lopes report no conflicts of interest. J. Xin Liao was supported by a research fellowship granted by the Department of Veterans Affairs Office of Academic Affiliations, Washington, DC, United States of America.
Compliance with Ethics Guidelines
This study was approved by the Veterans Affairs Central Institutional Review Board (reference # 18-33) with a waiver of informed consent. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
The study data may be made available upon request and approval by the Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liao, J.X., Appaneal, H.J., Menon, A. et al. Decreasing Antibiotic Resistance Trends Nationally in Gram-Negative Bacteria Across United States Veterans Affairs Medical Centers, 2011–2020. Infect Dis Ther 12, 1835–1848 (2023). https://doi.org/10.1007/s40121-023-00827-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-023-00827-9