Abstract
Purpose
The aim was to analyse the clinical and economic impact of carbapenemase-producing Enterobacterales (CPE) infections.
Methods
Case–control study. Adult patients with CPE infections were considered cases, while those with non-CPE infections were controls. Matching criteria were age (± 5 years), sex, source of infection and microorganism (ratio 1:2). Primary outcome was 30-day mortality. Secondary outcomes were 90-day mortality, clinical failure, hospitalisation costs and resource consumption.
Results
246 patients (82 cases and 164 controls) were included. Klebsiella pneumoniae OXA-48 was the most common microorganism causing CPE infections. CPE cases had more prior comorbidities (p = 0.007), septic shock (p = 0.003), and were more likely to receive inappropriate empirical and definitive antibiotic treatment (both p < 0.001). Multivariate analysis identified septic shock and inappropriate empirical treatment as independent predictors for 7-day and end-of-treatment clinical failure, whereas Charlson Index and septic shock were associated with 30- and 90-day mortality. CPE infection was independently associated with early clinical failure (OR 2.18, 95% CI, 1.03–4.59), but not with end-of-treatment clinical failure or 30- or 90-day mortality. In terms of resource consumption, hospitalisation costs for CPE were double those of the non-CPE group. CPE cases had longer hospital stay (p < 0.001), required more long-term care facilities (p < 0.001) and outpatient parenteral antibiotic therapy (p = 0.007).
Conclusions
The CPE group was associated with worse clinical outcomes, but this was mainly due to a higher comorbidity burden, more severe illness, and more frequent inappropriate antibiotic treatment rather than resistance patterns as such. However, the CPE group consumed more healthcare resources and incurred higher costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence and spread of multidrug-resistant (MDR) bacterial infections has become a major public health concern, as these bacteria are prone to human-to-human transmission and are difficult to treat [1]. They have traditionally been linked to healthcare settings [2], where they can be responsible for outbreaks among patients, but the problem is further exacerbated by reports in recent years of an increase in community settings [3]. In relation to the healthcare sector, the spread of MDR bacteria is expected to cause over 10 million deaths per year by 2050, and in terms of global economic output, production losses are estimated at billions of dollars [4].
In recent years, enzyme-producing Enterobacterales that confer resistance to beta-lactam antibiotics have appeared and spread all around the world [1, 4, 5]. This includes carbapenemase-producing Enterobacterales (CPE), resistant to carbapenem antibiotics, the traditional cornerstone for the treatment of severe infections caused by gram-negative bacteria [4, 5].
The 2022 European Centers for Disease Prevention and Control (ECDC) annual epidemiological report showed that 10.9% of Klebsiella pneumoniae isolates were carbapenem-resistant, with an almost 50% increase between 2019 and 2022 [6]. Not surprisingly, the World Health Organization (WHO) has declared antibiotic resistance as one of the 10 public health threats facing humanity, and CPE as one of the critical pathogens for which new antibiotics are urgently needed [7, 8].
Infections caused by CPE are difficult to treat because there are few options available due to their extensive resistance profile. Until quite recently, patients with CPE infections were treated with polymyxins or aminoglycosides in monotherapy or in combination with other antibiotics with suboptimal clinical results and high rates of toxicity [9, 10] With the development of novel drugs during the last years (such as ceftazidime-aztreonam, imipenem-relebactam, meropenem-vaborbactam, or cefiderocol) more antibiotic options are available for the management of CPE infections. These new drugs should be reserved for treatment of MDR gram negative bacteria with limited or no alternative options, with antimicrobial stewardship programs to limit the development of resistance to the novel antibiotic agents [11]. Despite the development of these novel antibiotics, treatment options for metallo beta-lactamase (MBL) mediated resistance patterns are limited. Although previous studies [10, 12,13,14,15,16,17] have analysed the clinical and economic burden related to CPE infections, in the present work we assessed clinical response, mortality, resource consumption and economic impact as a whole to give a more comprehensive view of a global problem. In the present study, we hypothesised that patients admitted with active infections caused by CPE have worse clinical outcomes, consume more resources and incur higher economic costs compared to patients admitted with infections caused by susceptible or non-MDR bacteria. The aim of this study was to analyse the clinical and economic impact of CPE infections.
Patients and methods
Study design
This was a retrospective case–control study of patients with active infections caused by CPE and non-MDR bacteria between January 2010 and October 2022. The research was carried out at the Parc de Salut Mar, in Barcelona (Spain).
During this time, every positive culture from a clinical sample showing growth of CPE isolates was examined; only those patients with criteria for infection were included as cases. Patients with a positive culture but no signs of infection were considered as colonised and excluded. Inclusion criteria were age 18 years or older, isolation of CPE in a clinical sample, and signs and symptoms of infection in the same focus as the culture. Outpatients were only included if they required hospitalisation due to the infection. Only the first episode of infection per patient was considered. Exclusion criteria were: (i) polymicrobial infections, (ii) colonisation without active infection, and (iii) end-of-life patients (palliative care patients) who did not receive active therapy for the infection.
The control group was made up of patients with active non-MDR bacterial infections. The matching criteria were based on controls and cases sharing a susceptible strain of the same bacterial species, focus of infection, and same sex and age ± 5 years.
As the initial control groups did not reach the sample size required for statistical analysis when all four matching criteria were combined (see further), the requirement for control inclusion was relaxed to both the same focus of infection and susceptible strain of the same microorganism. Matching by age and/or sex was applied whenever possible.
Sample size determination was based on the results of a previous study [18] to detect a 10% difference in 30-day mortality between CEP and non-MDR bacteria; statistical power was set at 80% and alpha error at 0.05. The sample size ratio for matching was 1:2 and 82 cases and 164 controls were obtained.
Patients were followed for 90 days from the onset of the infection. The follow-up was carried out through the electronical clinical records, which integrates hospital, primary care, homecare, long-term facilities, and social services records in the healthcare region of the hospital setting.
Outcomes
The primary outcome variable was all-cause mortality at 30 days, considering day 1 as the first day of positive culture. Secondary outcomes were clinical failure at day 7 (early clinical failure) and end of treatment, crude mortality at day 90, hospitalisation costs and resource consumption.
Data collection, variables, and definitions
Information on the study variables was obtained from an electronic medical record and economic data through the hospital’s economic database.
The variables considered for the clinical impact study were age, gender, underlying diseases according to the Charlson comorbidity index [19], antibiotic treatment in the previous 3 months, hospitalisation, invasive devices (urinary catheter, vascular access) or dialysis, microorganism isolated, source of infection, empirical and definitive treatment, duration, and antibiotic-related side effects.
An assessment of clinical severity was made for septic shock and intensive care unit (ICU) admission in the first 72 h, as well as a Sequential Organ Failure Assessment (SOFA) [20]. Septic shock was defined according to the Sepsis-3 definition [21]. In case of bacteraemia, the Pitt score [22] was applied.
Clinical failure was defined as persistence or worsening of signs and/or symptoms of the infection and/or death. Empirical antibiotic therapy was considered appropriate when at least one antibiotic active in vitro active antibiotic was given against the microorganism was administered.
For the consumption of healthcare resources, several variables were considered: length of stay, need for outpatient parenteral antibiotic treatment and long-term care facilities, and readmissions at 90 days of follow-up. Finally, the overall cost of hospitalisation, pharmacy and diagnostic tests for each patient were also assessed.
Microbiological data
MDR was defined as resistance to at least one agent in three or more antimicrobial categories for each organism, according to current standard definitions [23]. Non-MDR was considered when resistance to fewer than three active antimicrobial categories was observed. According to this classification, the antimicrobial categories used to define MDR in Enterobacteriales were: aminoglycosides, anti-MRSA cephalosporins, antipseudomonal penicillins + β-lactamase inhibitors, carbapenems, 1st and 2nd generation cephalosporins, 3rd and 4th generation cephalosporins, cephamycins, fluoroquionolones, folate pathway inhibitors (trimethoprim-sulphamethoxazole), glycylcyclines (tigecycline), monobactams, penicillins + β-lactamase inhibitors, phenicols (chloramphenicol), phosphonic acids, polymyxins and tetracyclines [19].
Routine identification and susceptibility testing of causative microorganisms was performed using automated systems (the Vitek-2® [BioMérieux] for blood cultures, and the MicroScan® WalkAway [Beckman-Coulter] for other sample types). Results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards in force at the time of culture. Carbapenemases were identified by multiplex PCR assay, using the LightMix® Modular Carbapenemase panel (Roche Diagnostics).
Statistical analysis
Qualitative variables were shown as numbers and percentages, and quantitative variables as median and interquartile range (IQR). Quantitative variables were analysed using the Student’s t-test or Mann–Whitney U test, and qualitative variables using the Chi-Square (χ2) or Fisher’s exact test, as appropriate.
A logistic regression model was used to examine variables associated with clinical failure at day 7 and at end of treatment, expressing the results as odds ratio (OR) and 95% confidence interval (CI).
Kaplan–Meier survival curves and the log-rank test were used to detect differences in 30- and 90-day mortality.
The Cox proportional hazards model was used to perform multivariate survival analyses for 30- and 90-day mortality. Results were expressed as hazard ratio (HR) and 95% CI. The proportional hazards assumption was tested.
To control for confounding, variables in the crude analysis that were associated with exposure (p ≤ 0.20) or outcome (OR or HR < 0.65 or > 1.5), and those that were not considered to be intermediate variables between exposure and outcome were candidates for multivariate analysis. Variables leading to substantial confounding if removed (10% or more change in the coefficient estimate) were retained in the model, along with exposure (CPE group). Manually selected, backward stepwise regression was applied. Variables with > 25% missing values were not considered in multivariate analysis.
For the economic analysis, median regression (to deal with non normal dependent variables) was used. Results were expressed as the difference in medians (DM). Interpretation of the coefficients was based on the difference of means, as it was for the interpretation of coefficients in multiple linear regression.
All p-values were 2-tailed and statistical significance was set at ≤ 0.05. Statistical analyses were performed using STATA 15.1. STROBE guidelines were used for the reporting of the study (Supplementary Table S1).
Ethics
The Clinical Research Ethical Committee of the Parc Salut Mar approved this study (registration no. 2022/1046). Written informed consent was waived due to its observational and retrospective nature.
Results
A total of 246 patients eligible for inclusion in the study were studied: 82 infections were caused by CPE (cases) and 164 by non-MDR Enterobacterales (controls) (Fig. 1).
Of the CPE isolates, 58 (70.73%) were oxacillin-type carbapenemases (OXA-48); 16 (19.51%) were Verona integron–encoded metallo-β-lactamase (VIM), and 4 (4.88%) each were Klebsiella pneumoniae carbapenemase (KPC) and New Delhi metallo-β-lactamase (NMD). In terms of antimicrobial susceptibility, all CPE isolates were susceptible to colistin. Of the OXA-48 tested: 22/23 (95.7%) were susceptible to ceftazidime-avibactam, 35/49 (71.4%) to amikacin, 18/28 (64.3%) to fosfomycin, 18/57 (31.6%) to trimethoprim-sulfamethoxazole and 7/57 (12.3%) to ciprofloxacin. Susceptibility rates were lower for VIM isolates: 0/3 (0%) to ceftazidime-avibactam, 8/14 (57.1%) to amikacin, 1/15 (6.7%) to trimethoprim-sulfamethoxazole, 1/16 (6.3%) to ciprofloxacin, (1/16, 6.3%), except against fosfomycin (7/8, 87.5%). All KPC and NMD strains were resistant to ciprofloxacin. Susceptibility to fosfomycin was found in 66.7%,of NDM, and to trimethoprim-sulfamethoxazole in 25% of NDM and KPC.
Demographic and epidemiological information, clinical features, and therapeutic management
Differences in baseline characteristics, severity assessments and therapeutic management are shown in Table 1. Considering the matching criteria (age, sex, microorganism, and site of infection) there were no differences among between the studied.
The most frequently isolated microorganisms and sites of infection can be seen in Fig. 2(A/B). Overall, 20% of patients had bloodstream infections: 30 (18.3%) in the non-MDR group and 20 (24.4%) in the CPE cases (p = 0.263).
Distribution of microorganisms (A) and sources of infection (B). *Distribution of microorganisms (non-MDR vs CPE group): Escherichia. coli 18 (11%) vs 9 (11%); Klebsiella pneumoniae 112 (68.3%) vs 56 (68.3%); Enterobacter cloacae 24 (14.6%) vs 12 (14.6%); Citrobacter freundii 6 (3.7%) vs 3 (3.7%) and Klebsiella oxytoca 4 (2.4) vs 2 (2.4%). All p values = 1. Abbreviations: MDR (MDR (multidrug-resistant), CPE (carbapenemase-producing Enterobacterales). *Distribution of source of infection (non-MDR vs CPE group): Respiratory 40 (24.4%) vs 20 (24.4%), p = 1; UTI 70 (42.7%) vs 34 (41.5%), p = 0.855; SSTIs 22 (13.4%) vs 11 (13.4%), p = 1; Catheter 1 (0.6%) vs 3 (3.7%), p = 0.109; IAI 18 (11%) vs 10 (12.2), p = 0.776 and primary bacteraemia 13 (7.9) vs 4 (4.9%), p = 0.374. Abbreviations: MDR (MDR (multidrug-resistant), CPE (carbapenemase-producing Enterobacterales), UTI (Urinary Tract Infection, includes pyelonephritis, prostatitis and urosepsis), SSTIs (Skin and Soft Tissue Infections, includes cellulitis, abscess, myositis and fasciitis), IAI (Intra-abdominal Infections, includes Clostridioidesum difficile Clostridioides difficile, gastrointestinal tract infection and necrotising enterocolitis)
CPE patients had more underlying diseases as assessed by the Charlson comorbidity index than those with non-MDR bacteria (median points, 4 [IQR 2–6] vs. 3 [IQR 1 to 5]; p = 0.007). Dementia, lung, kidney and liver diseases were seen more in the CPE group.
CPE cases were more related to healthcare activity [previous hospital stays (p < 0.001), previous antibiotic exposure (p < 0.001) and previous use of invasive devices (p < 0.001) in the previous 3 months; had more hospital-acquired infections (p = 0.045) and were more likely to be previously colonised by an MDR microorganism (p < 0.001).
At the onset of infection, CPE patients were more seriously ill, reflected in a higher proportion of septic shock (p = 0.003), ICU stay (p = 0.008), and higher SOFA and Pitt scores (p < 0.001 in both cases).
CPE cases were more likely to receive inappropriate antibiotic treatment (p < 0.001), have longer courses of antibiotic therapy (p < 0.001) and more antibiotic-related side effects (p < 0.001). Most common target antibiotic regimens used for CPE infections included: ceftazidime-aztreonam in monotherapy (18 patients) or in combination with aminoglycosides or polymyxins (4 patients); tigecycline in monotherapy (3 patients) or tigecycline in combination with carbapenems, polymyxins, aminoglycosides and/or fluoroquinolones (10 patients); colistin in monotherapy (3 patients) or in combination with carbapenems, fosfomycin or others (6 patients) and fosfomycin in monotherapy (5 patients).
Clinical outcomes
CPE infections had higher rates of clinical failure at day 7 (79.3 vs. 39%; p < 0.001) and at the end of treatment (20.7% vs. 4.3%; p < 0.001), as well as higher crude mortality at 90 days (25.6% vs. 14.6% p = 0.036). However, no differences were observed for 30-day mortality (11% vs. 6.1%, p = 0.117). Higher 30-day mortality rates were observed for CPE cases with pneumonia or bacteraemia (20%) or septic shock (40%). The microbiological clearance rate at day 90 was higher in non-MDR infections (n = 69/79; 90.8 vs. n = 32/45; 71.1%; p = 0.005).
Table 2 shows the multivariate analysis of clinical failure at day 7 and at the end of treatment. CPE infection was independently associated with clinical failure at day 7 (adjusted OR 2.18; CI 1.03–4.59; p = 0.041) but not at the end of treatment (Table 2).
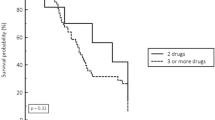
Figure 3a and b show the respective Kaplan–Meier curves for 30- and 90-day mortality by groups. After adjusting for confounders, CPE infection was not associated with either 30-day or 90-day mortality (Table 3).
Resource consumption and costs
Compared with the non-MDR group, patients with CPE infection had longer hospital stays (median days, 35.5 [IQR 16–60] vs. 18 [10–38.5]; p < 0.001), more frequently needed long-term care facilities (p < 0.001) and outpatient parenteral antibiotic therapy (p = 0.007).
With respect to the economic burden, the costs of hospitalisation, pharmacy, and diagnostic tests were all higher in CPE patients (all p < 0.001). Hospitalisation costs for CPE patients were more than double those associated with non-MDR cases, with a median difference of 13,417.38 € between groups (p < 0.001) (Table 4). These results were also confirmed after adjusting for confounders (Table 5).
Discussion
Following the WHO alert that CPE are becoming a major public health concern [7, 8], more studies are being conducted to gain a better understanding of this type of infection and to evaluate its impact. This case–control study evaluated the burden of CPE infections on clinical and economic outcomes.
CPE infections did not result in increased mortality at 30 or 90 days, or in higher clinical failure rates at the end of antibiotic treatment. On the other hand, CPE cases were independently associated with higher early clinical failure.
Conflicting results have been published on the impact of CPE infections on clinical outcomes [12,13,14,15]. Apart from carbapenem resistance itself, other factors, such as patient comorbidities, host response, source and severity of the infection, therapeutic management, type of microorganism, mechanism of carbapenem resistance and virulence factors, are likely to play a role in these discrepancies.
In our cohort, the overall 30-day mortality in CPE cases was 11%, which is a lower proportion than has been previously reported [13, 15, 24, 25]. However, mortality was higher in patients with pneumonia or bacteraemia (20%) and in those with septic shock (40%). In addition, more than 40% of included patients had a urinary source, with a mortality rate of 6% in this subgroup.
Baseline comorbidities and septic shock at the infection were found to be predictors of 30- and 90-day mortality, as previously reported [12,13,14,15, 25]. Although inappropriate antibiotic therapy was not independently associated with higher mortality, it was independently associated with increased clinical failure. CPE cases were three times more likely than non-MDR cases to receive inappropriate empiric and definitive antibiotic therapy (91.4% vs. 31.1% and 19.5% vs 4.5%, respectively; both p < 0.001). Given that the presence of CPE substantially increases the risk of receiving inappropriate antibiotic therapy and that this single factor is relatively modifiable, active surveillance and use of local data should be applied to guide treatment decisions and improve outcomes in CPE infections.
In terms of resource consumption, patients with CPE infection required longer hospital stays and more frequently used long-term care facilities and outpatient parenteral antibiotic therapy, which resulted in significantly higher costs for hospitalisation, pharmacy, and diagnostic tests. These findings are consistent with previous reports [12, 26,27,28,29]. Furthermore, CPE cases were more likely to receive longer courses of antibiotics and to have more antibiotic-related side effects. Following on from this, due to the susceptibility profile of CPE infections, there were few, if any, opportunities to use oral antibiotic treatment in our cohort, which perpetuates the vicious cycle of longer length of stay or need for outpatient parenteral antibiotic therapy and translates into higher costs, both of which could be avoided in non-MDR cases.
In general, a patient with a CPE infection should be considered as more complex, vulnerable, and costly. It should raise alarms in the healthcare system, since these patients have more comorbidities and severe illnesses, which involves a greater economic burden and special measures (such as specific environmental cleanup cleaning activities of the environment, isolation rooms and consumables, faecal and medical waste management, staff education). Infection control measures are urgently needed to reduce the selection and spread of these bacteria.
This study has some limitations. First, data were collected retrospectively from medical charts, which makes the results susceptible to selection and reporting biases. We cannot exclude the possibility that there were residual confounding factors not taken into account that could have influenced the results. Second, a single hospital centre does not represent all CPE infections. In our study, K. pneumoniae OXA-48 was the most frequent causative microorganism of CPE infections, which is consistent with epidemiological studies in Spain [30, 31], but these results are not transferable to settings with a different epidemiology. Third, the sample size precluded analysis of certain subgroups, such as bacterial genus, species and carbapenemase type or the source of infection. Fourth, although considerable efforts were made to adjust for four matching criteria, not all included patients were fully matched. However, no statistically significant differences in matching characteristics were observed between groups. Fifth, our study was conducted between 2010 and 2022, and therefore, during the first years of the study novel β-lactam/β-lactamase inhibitor combinations or cefiderocol were not available. This could have conditioned a worse outcome due to the use of alternative treatments (such as polymyxins), less effective and with more adverse events. However, as the access to new antibiotics is not homogenous, we consider that our results are also of interest since in some settings patients are still managed with antibiotic combinations of “old drugs”. Finally, the inclusion of nosocomial CPE infections may have overestimated the total costs of hospitalisation in this group. The estimation of costs attributable to these infections is methodologically challenging since they may be biased by the initial reason for admission. After using multivariate analysis to reduce this bias, the CPE cases were still more costly.
Our study has several strengths. First, only infected patients were included, unlike other cohorts in which colonised cases were also taken into account [14]. In keeping with this, about 20% of patients in each group had a bloodstream infection or septic shock, which is a good indicator that the patients were appropriately selected. Second, only CPE were assessed, and not carbapenem-resistant isolates, in which underlying resistance mechanisms other than carbapenemase production may have been involved. Third, several outcomes were considered (clinical failure, mortality, resource consumption, and economic burden), to give a more comprehensive view of this problem. Finally, the inclusion of a heterogeneous sample of CPE reflects the day-to-day reality of hospitals.
In conclusion, this study found that patients with CPE infections had worse clinical outcomes, although this was explained by higher patient comorbidity, more frequent inappropriate empirical treatment, and more severe infection at the onset of infection, not by antibiotic resistance per se. Nevertheless, CPE infections consumed more healthcare resources and the overall costs of hospitalisation were higher.
Key points
-
Carbapenemase-producing Enterobacterales (CPE) infections are increasing worldwide.
-
The clinical and economic burden of CPE infections was evaluated.
-
CPE was related to sicker patients, inappropriate treatment and early clinical failure.
-
CPE cases had longer hospital stay and higher costs.
-
CPE could be a marker of more complicated and costly admissions.
Data availability
Data will be made available upon personal contact to authors.
References
Mills JP, Marchaim D. Multidrug-Resistant Gram-Negative Bacteria. Infect Dis Clin North Am 2021; 35:969–994. Available at: https://linkinghub.elsevier.com/retrieve/pii/S089155202100074X.
Magiorakos AP, Burns K, Rodríguez Baño J, et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control. Antimicrob Resist Infect Control 2017; 6:113. Available at: https://aricjournal.biomedcentral.com/articles/https://doi.org/10.1186/s13756-017-0259-z.
van Duin D, Paterson DL. Multidrug-Resistant Bacteria in the Community. Infect Dis Clin North Am 2020; 34:709–722. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0891552020300647.
van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017; 8:460–469. Available at: https://www.tandfonline.com/doi/full/https://doi.org/10.1080/21505594.2016.1222343.
Grundmann H, Glasner C, Albiger B, et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 2017; 17:153–163. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1473309916302572.
European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report 2022. Stockholm: ECDC; 2023.
Ten threats to global health in 2019. World Health Organization. Available at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
WHO. WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017.
Rodríguez-Baño J, Gutiérrez-Gutiérrez B, Machuca I, Pascual A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin Microbiol Rev 2018; 31. Available at: https://cmr.asm.org/content/31/2/e00079-17.
Giacobbe DR, Marelli C, Cattardico G, et al. Mortality in KPC-producing Klebsiella pneumoniae bloodstream infections: a changing landscape. J Antimicrob Chemother 2023; 78:2505–2514. Available at: https://academic.oup.com/jac/article/78/10/2505/7247462.
Suay-García, Pérez-Gracia. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019; 8:122. Available at: https://www.mdpi.com/2079-6382/8/3/122.
Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 2017; 17:279. Available at: http://bmcinfectdis.biomedcentral.com/articles/https://doi.org/10.1186/s12879-017-2383-z.
Tamma PD, Goodman KE, Harris AD, et al. Comparing the Outcomes of Patients With Carbapenemase-Producing and Non-Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae Bacteremia. Clin Infect Dis 2017; 64:257–264. Available at: https://academic.oup.com/cid/article/64/3/257/2452664.
Saito S, Hayakawa K, Tsuzuki S, et al. A Matched Case-Case-Control Study of the Impact of Clinical Outcomes and Risk Factors of Patients with IMP-Type Carbapenemase-Producing Carbapenem-Resistant Enterobacteriaceae in Japan. Antimicrob Agents Chemother 2021; 65. Available at: https://journals.asm.org/doi/https://doi.org/10.1128/AAC.01483-20.
Seo H, Lee SC, Chung H, et al. Clinical and Microbiological Analysis of Risk Factors for Mortality in Patients with Carbapenem-Resistant Enterobacteriaceae Bacteremia. Int J Antimicrob Agents 2020; 56:106126. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0924857920303095.
Otter JA, Burgess P, Davies F, et al. Counting the cost of an outbreak of carbapenemase-producing Enterobacteriaceae : an economic evaluation from a hospital perspective. Clin Microbiol Infect 2017; 23:188–196. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1198743X16304645.
Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths Attributable to Carbapenem-Resistant Enterobacteriaceae Infections. Emerg Infect Dis 2014; 20:1170–1175. Available at: http://wwwnc.cdc.gov/eid/article/20/7/12-1004_article.htm.
Zhang H, Guo Z, Chai Y, et al. Risk Factors for and Clinical Outcomes of Carbapenem-Resistant Klebsiella pneumoniae Nosocomial Infections: A Retrospective Study in a Tertiary Hospital in Beijing, China. Infect Drug Resist 2021; Volume 14:1393–1401. Available at: https://www.dovepress.com/risk-factors-for-and-clinical-outcomes-of-carbapenem-resistant-klebsie-peer-reviewed-article-IDR.
Charlson ME, Pompei P, Ales KL, MacKenzie RC. A new method of classifying prognostic in longitudinal studies: development. J Chronic Dis. 1987;40:373–83.
Jones AE, Trzeciak SKJ. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2010;37:1649–54.
Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47:1181–1247. Available at: https://link.springer.com/https://doi.org/10.1007/s00134-021-06506-y.
Rhee JY, Kwon KT, Ki HK, et al. Scoring systems for prediction of mortality in patients with intensive care unit-acquired sepsis: A comparison of the PITT bacteremia score and the acute physiology and chronic health evaluation II scoring systems. Shock. 2009;31:146–50.
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
Ramos-Castañeda JA, Ruano-Ravina A, Barbosa-Lorenzo R, et al. Mortality due to KPC carbapenemase-producing Klebsiella pneumoniae infections: Systematic review and meta-analysis. J Infect 2018; 76:438–448. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0163445318300604.
So-ngern A, Osaithai N, Meesing A, Chumpangern W. Mortality rate and factors associated with mortality of carbapenem-resistant Enterobacteriaceae infection. Drug Target Insights 2023; 17:120–125. Available at: https://journals.aboutscience.eu/index.php/dti/article/view/2622.
Bartsch SM, McKinnell JA, Mueller LE, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect 2017; 23:48.e9–48.e16. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1198743X16303895.
Thaden JT, Li Y, Ruffin F, et al. Increased costs associated with bloodstream infections caused by multidrug-resistant gram-negative bacteria are due primarily to patients with hospital-acquired infections. Antimicrob Agents Chemother. 2017;61:1–10.
Huang W, Qiao F, Zhang Y, et al. In-hospital Medical Costs of Infections Caused by Carbapenem-resistant Klebsiella pneumoniae. Clin Infect Dis 2018; 67:S225–S230. Available at: https://academic.oup.com/cid/article/67/suppl_2/S225/5181281.
Neidell MJ, Cohen B, Furuya Y, et al. Costs of Healthcare- and Community-Associated Infections With Antimicrobial-Resistant Versus Antimicrobial-Susceptible Organisms. Clin Infect Dis 2012; 55:807–815. Available at: https://academic.oup.com/cid/article-lookup/doi/https://doi.org/10.1093/cid/cis552.
Cañada-García JE, Moure Z, Sola-Campoy PJ, et al. CARB-ES-19 Multicenter Study of Carbapenemase-Producing Klebsiella pneumoniae and Escherichia coli From All Spanish Provinces Reveals Interregional Spread of High-Risk Clones Such as ST307/OXA-48 and ST512/KPC-3. Front Microbiol 2022; 13. Available at: https://www.frontiersin.org/articles/https://doi.org/10.3389/fmicb.2022.918362/full.
Vázquez-Ucha JC, Seoane-Estévez A, Rodiño-Janeiro BK, et al. Activity of imipenem/relebactam against a Spanish nationwide collection of carbapenemase-producing Enterobacterales. J Antimicrob Chemother 2021; 76:1498–1510. Available at: https://academic.oup.com/jac/article/76/6/1498/6157431.
Acknowledgements
We would like to thank Francesc Cots Reguant and Enric García-Alzorriz Morral for providing the economic data.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. To disclose and have received no funding for this study.
Author information
Authors and Affiliations
Contributions
I.L.M: Conceptualization, data curation, formal analysis, investigation, resources, methodology and statistical analyses, writing-original draft, writing-review and validation. A.C.C: investigation, data curation, statistical analyses and writing-original draft. M.M.M, L. S.R, A.S.P and S.E.C: Investigation, resources, writing-review and validation. X.D-J. formal analysis, methodology, statistical analyses, writing-review and validation. S.G-Z and J. P.H: Conceptualization, methodology, formal analysis, writing-review, editing and validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López Montesinos, I., Carot-Coll, A., Montero, M.M. et al. A case–control study of the clinical and economic impact of infections caused by Carbapenemase-producing Enterobacterales (CPE). Infection (2024). https://doi.org/10.1007/s15010-024-02268-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-024-02268-z