Abstract
Purpose
Sepsis has a high incidence and a poor prognosis. Early recognition is important to facilitate timely initiation of adequate care. Sepsis screening tools, such as the (quick) Sequential Organ Failure Assessment ((q)SOFA) and National Early Warning Score (NEWS), could help recognize sepsis. These tools have been validated in a general immunocompetent population, while their performance in immunocompromised patients, who are particularly at risk of sepsis development, remains unknown.
Methods
This study is a post hoc analysis of a prospective observational study performed at the emergency department. Inclusion criteria were age ≥ 18 years with a suspected infection, while ≥ two qSOFA and/or SOFA criteria were used to classify patients as having suspected sepsis. The primary outcome was in-hospital mortality.
Results
1516 patients, of which 40.5% used one or more immunosuppressives, were included. NEWS had a higher prognostic accuracy as compared to qSOFA for predicting poor outcome among immunocompromised sepsis patients. Of all tested immunosuppressives, high-dose glucocorticoid therapy was associated with a threefold increased risk of both in-hospital and 28-day mortality.
Conclusion
In contrast to NEWS, qSOFA underestimates the risk of adverse outcome in patients using high-dose glucocorticoids. As a clinical consequence, to adequately assess the severity of illness among immunocompromised patients, health care professionals should best use the NEWS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a life-threatening syndrome of organ dysfunction caused by a dysregulated host response to infection that affects 50 million patients worldwide annually [1]. Despite efforts to improve recognition and treatment, sepsis remains an important cause of morbidity and mortality among patients admitted to the hospital with mortality rates ranging from 20 to 36% [2,3,4,5,6,7]. Timely initiation of adequate treatment is essential to prevent clinical deterioration. Therefore, the key to reducing sepsis-related mortality could lie in improving early recognition and identification of those at risk of poor outcomes [7, 8]. Currently, the most commonly used clinical assessment tools to facilitate early recognition of sepsis are the Systemic Inflammatory Response Syndrome (SIRS, Sepsis-2) criteria, (quick) Sequential Organ Failure Assessment ((q)SOFA, Sepsis-3), and the National Early Warning Score (NEWS) [1]. Of these scores, the NEWS and SOFA appears to have the highest accuracy to predict sepsis-related mortality [9,10,11]. Although the NEWS was not specifically developed for early sepsis recognition, it can accurately identify patients with more severe disease and thereby facilitate early recognition of sepsis [9, 10].
Most research validating qSOFA and NEWS are performed among general emergency department (ED) and ICU patients—not explicitly assessing the scores in immunocompromised patients. Yet, immunocompromised patients with an infection are at increased risk for sepsis development. Early recognition of sepsis and risk prediction in immunocompromised patients might be more difficult due to their altered immune response. Data describing the prognostic accuracy of the qSOFA and NEWS in immunocompromised patients are scarce and are either contradicting or cannot be generalized to pharmacologically immunocompromised patients. Immunodeficiency due to co-morbidity (i.e., cancer, neutropenia, AIDS) is a risk for 28-day mortality among patients with sepsis admitted to the ICU [2]. Unexpectedly, patients with bacteremia after solid organ transplantation have a lower 28-day and 90-day mortality as compared to age-, sex-, and hospital-matched bacteremia patients without history of organ transplantation [12]. The performance of SIRS, qSOFA, and NEWS has been assessed in patients with a suspected infection after hematopoietic cell transplantation. In this population, the NEWS had a moderate sensitivity (78%) and specificity (70%), which outperformed qSOFA and SIRS [13]. Together, immunosuppression due to co-morbidity affects outcome in patients with sepsis, with relevance to the predictive performance of commonly used sepsis scores.
The effect of immunosuppressive medication on clinical outcomes in patients with sepsis, and thereby, the predictive performance of sepsis scores remains unknown. We hypothesized that dampening the immune response by immunosuppressive drugs affects the prognostic accuracy of NEWS and qSOFA, which is likely to depend on drug class. For this study we used the NEWS in addition to the qSOFA, as advised by the Dutch sepsis guideline to support sepsis recognition at the ED [14]. To this end, we compared the prognostic performance of NEWS and qSOFA to predict the in-hospital mortality in pharmacologically immunocompromised patients with sepsis at the ED, adjusted to the immunosuppressive drug class.
Methods
Study design
This is a post hoc analysis of a prospective observational cohort study of adult patients visiting the ED of the University Medical Center Groningen (UMCG), a tertiary medical center. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) criteria recommendation for cohort studies [15, 16]. The Dutch Medical Research Involving Human Subjects Act is not applicable for this study, as ruled by the Institutional Review Board of the University Medical Center Groningen, and a waiver was granted (METc 2015/164). Written informed consent was obtained from all patients included in this study.
Population
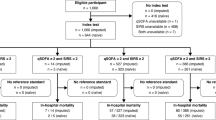
From March 2016 to July 2020, 39,719 adult patients (≥ 18 years of age) visiting our ED for internal medicine, gastroenterology, rheumatology, emergency medicine (non-trauma), or pulmonology, of which 31,331 visits occurred between 8:00 and 23:00 h, were screened for inclusion. Patients were only screened between 8:00 and 23:00 due to the availability of the research team. Patients who presented with a suspected infection (as determined by the treating physician upon initial contact based on focal symptoms suggestive of an infection [e.g., productive cough, dyspnea, dysuria, pollakiuria, abdominal pain, erythema]) and/or fever (≥ 38 °C, either at home or upon triage in the ED) were included by a trained team of researchers. This approach allowed to include data from a heterogeneous, real-world population of ED visitors with a clinically relevant infection, including patients with sepsis. Patients with an alternative non-infectious diagnosis upon ED visit (n = 50) were excluded, as were patients with missing outcome or stratification data (use of immunosuppressives, NEWS or (q)SOFA score) (n = 272). Thereby, 1516 subjects were included in the dataset for the current analysis (Fig. 1).
Data collection
Collected data included demographic characteristics, vital parameters, and laboratory measurements at admission. Patient characteristics consisted of age, sex, history of diabetes mellitus, chronic kidney disease, kidney transplantation, cardiovascular disease (defined as chronic heart failure and/or ischemic heart disease), and active cancer (defined as radiotherapy or chemotherapy treatment received up to 2 years prior to the current hospitalization). Data were collected from the electronic patient files, by shadowing and observing, as well as by interviewing patients and physicians. Data about treatment which was given outside the ED had not been collected. Information regarding use of immunosuppressive agents was subtracted from the patient’s medical record. The dosage of glucocorticoids was transformed into the equivalent prednisone dosage, based on the defined daily dose (DDD) as published by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology.
Outcomes
The primary outcome was in-hospital mortality, while secondary outcomes were ICU admission and 28-day mortality. Patients were allocated to the immunosuppressives group when using immunosuppressive drugs at the time of presentation to the ED [17]. Table S1 in the appendix provides an overview of the drugs included in each class. The relative high number of patients using glucocorticoids allowed us to subdivide the groups based on the dosage, being < 7.5 mg/day (low dose), 7.5–15 mg/day (intermediate dose), and ≥ 15 mg/day (high dose).
Statistical analysis
Statistical analyses were performed using R studio version 3.5.1 (RStudio, Inc., Boston, MA, USA). The following packaging were used: haven, lattice, ggplot2, ggpubr, ggsignif, tableone, cowplot, readxl, and GGally. Differences between two groups were compared using a two-tailed independent sample t-test, if normally distributed. In case of non-normally distributed data, a Mann–Whitney U test was used. Categorical variables were compared using a Chi-square test. In all cases, p < 0.05 was considered as a significant difference. Multiple univariate regression analyses were used to explore the association between the class of immunosuppressive drug use, adjusted for demographic and medical factors, and the different outcomes as specified above. Variables were entered in multivariate regression and were forward: conditional selected. To determine the predictive accuracy for the different scores, we calculated sensitivity, specificity, area under the receiver operating curve (AUROC), and Brier scores. We created confidence intervals by bootstrapping 2000 samples. Differences in AUROCs between nested models were tested using the approach of Robin et al. [18].
Results
Patient characteristics
We included 1516 patients with a median age of 64 years, of which 42.5% were female (Table 1). Overall, 53.1% of the patients had a diagnosis of sepsis according to the Sepsis-3 criteria (SOFA or qSOFA ≥ 2) (Table 1). The population was subdivided by the use of immunosuppressives. In total 614 (40.5%) patients used one or more immunosuppressive drugs, of which glucocorticoids were the most used: the cohort consisted of 496 (32.7%) glucocorticoid users (Table S1, appendix). Of the glucocorticoid users, 237 (15.6%) used < 7.5 mg/day, 117 (7.7%) used 7.5–15 mg/day, and 142 (9.4%) used > 15 mg/day. Other immunosuppressives used were TNF blockers (1.3%), cellular immunosuppressives (12.0%), selective immunosuppressives (1.8%), IMPDH inhibitors (11.2%), purine antagonist (3.8%), and folic acid antagonist (2.4%) (Table S2, appendix). According to the Sepsis-3 criteria, sepsis was more prevalent among patients using 0–15 mg glucocorticoids per day (64.6–68.4%) and patients using IMPD inhibitors (61.8%) as compared to the whole cohort (53.1%, Table S2, appendix).
Outcomes of sepsis in patients on immunosuppressives
In total, 77 (5.1%) of the patients were admitted to the ICU, 55 (3.6%) died in hospital, and 94 (6.2%) died within 28 days after the ED visit. Both the in-hospital and 28-day mortality rates were higher for patients using high-dose (> 15 mg/day) glucocorticoids, being 8.5% and 12.0%, respectively, while the number of patients admitted to the ICU did not differ (Table 1). Univariate analysis demonstrated a threefold increased risk of in-hospital and 28-day mortality among high-dose glucocorticoid users (Tables 2 and S3). For low- and intermediate-dose glucocorticoids, the in-hospital mortality rate, 28-day mortality rate and ICU admission rate did not differ from the overall population (Table 1). Use of either low- or intermediated dose glucocorticoids was not associated with increased risk of ICU admission, in-hospital or 28-day mortality (Tables 2, S3, and S4). The in-hospital mortality rate, 28-day mortality rate and ICU admission rate did not differ between patients using other classes of immunosuppressive drugs, except an unexpected lower 28-day mortality among patients using IMPDH inhibitors (Table S2). Folic acid antagonist use was associated with an increased risk of ICU admission, but not in-hospital or 28-day mortality (Tables 2, S3, and S4). In summary, use of high-dose glucocorticoids (> 15 mg/day) was associated with increased risk of both in-hospital and 28-day mortality, use of IMPD inhibitors was associated with a lower 28-day mortality, and folic acid antagonist use was associated with an increased risk of ICU admission.
Sepsis screening tools in patients using glucocorticoids
To further explore the predictive performance of qSOFA and NEWS in patients using glucocorticoids, we first investigated the difference in the scores at baseline. The SOFA score was higher in patients with low and intermediate dose of glucocorticoids compared to the rest of the cohort. The SIRS and NEWS are lower in patients using low-dose glucocorticoids as compared to the rest of the cohort. No differences in sepsis screening tools were found between patients using high-dose glucocorticoids and patients without high-dose glucocorticoids (Table 1). The sepsis scores of patients using other types of immunosuppressives are depicted in the supplemental data (Table S2).
qSOFA
All individual qSOFA score items and use of high-dose glucocorticoids were univariately associated with an increased risk of in-hospital mortality (Table 3). Multivariate regression analysis showed that use of high-dose glucocorticoids was independently associated with an increased risk of in-hospital mortality (OR 3.22 [95% CI 1.56–5.99], p < 0.05; Table 3). Other factors predictive of in-hospital mortality in this model were systolic blood pressure < 100 mmHg and respiratory rate ≥ 22/min, but not consciousness (EMV < 15).
NEWS
Abnormal NEWS scores for respiratory rate (< 12 or > 20/min), oxygen suppletion and saturation (SpO2 < 96%), systolic blood pressure (< 111 or > 219 mmHg), heart rate (< 51 or > 90 bpm), and consciousness (EMV < 15) were univariately associated with in-hospital mortality (Table 4). When adjusting the model for use of high-dose glucocorticoids, we identified a threefold increased risk for in-hospital mortality among high-dose glucocorticoid users (OR 3.03 [95% CI 1.45–5.91], p < 0.05; Table 4), while oxygen suppletion and abnormal respiratory rate and systolic blood pressure are other independent predictors from the NEWS.
Comparing the performance of qSOFA and NEWS in patients using high-dose glucocorticoids
The sensitivity of qSOFA to predict 28-day mortality in the whole cohort was 25.4% and 25.0% for the immunocompromised (any class) and high-dose glucocorticoid group (Table 5). The specificity was 93.3% in the whole cohort, 92.4% in immunocompetent patients, 94.8% in immunocompromised patients, and 96.1% among patients using high-dose glucocorticoids. The sensitivity of the NEWS ranged from 65.4 to 100.0%, while the specificity was from 73.0 to 74.0% (Table 5). The sensitivity of the NEWS is higher among immunocompromised patients and those using high-dose glucocorticoids, whereas the specificity remained unaffected. The sensitivity of the SOFA ranged from 85.7 to 100.0%, while the specificity was from 40.4 to 51.5% (Table 5). The AUROC of the NEWS (0.752) to predict in-hospital mortality was higher than the qSOFA (0.683, p < 0.05, Fig. 2) among the whole study population. Specifically, among immunocompromised patients, the AUROC of NEWS (0.757) and SOFA (0.800) scores were higher as compared to the qSOFA (0.663, p < 0.05, Table 5, Fig. 2). In patients using high-dose glucocorticoids, the NEWS (0.858) and SOFA (0.899) were better in predicting in-hospital mortality as compared to qSOFA (0.707, p < 0.05, Table 5, Fig. 2).
ROC curve of qSOFA and NEWS and in-hospital mortality. The AUC was determined using a bootstrap with 2000 steps. qSOFA quick Sequential Organ Failure Assessment, NEWS National Early Warning Score, AUC area under the curve. A ROC curve in the overall population where NEWS (AUC: 0.752) had a higher AUC compared to qSOFA (AUC: 0.683) p < 0.05. B ROC curve in the immunocompromised population where NEWS (AUC: 0.757) had a higher AUC compared to qSOFA (AUC: 0.663) p < 0.05. C ROC curve in the high-dose glucocorticoids population where NEWS (AUC: 0.858) had a higher AUC compared to qSOFA (AUC: 0.707) p < 0.05
Discussion
Here, we evaluated the effect of immunosuppressive drugs on the performance of commonly used sepsis scores to predict clinically relevant outcomes of sepsis. Of all classes of immunosuppressives studied, only the use of high-dose glucocorticoids (> 15 mg/day) was associated with a profoundly increased risk of in-hospital mortality, which remained significant in multivariate analysis. When comparing the performance of qSOFA and NEWS in predicting in-hospital mortality and attempting to adjust for high-dose glucocorticoid use, we found that NEWS is better in predicting in-hospital mortality than the qSOFA among immunocompromised patients.
A common concern in patients on immunosuppressive agents is a blunted immune response leading to a lack of clinical symptoms that usually accompany sepsis [13]. Subsequently, early sepsis recognition can be more challenging in immunocompromised patients. In our cohort we found a sensitivity around 25% and specificity ranging from 92.4 to 96.1% for qSOFA to predict in-hospital mortality. The highest specificity of the qSOFA was found in patients on high-dose glucocorticoids. The sensitivity of qSOFA was slightly lower in our immunocompromised groups, being 25.0% versus 25.7% in immunocompetent patients. The qSOFA is known to suffer from a low sensitivity for predicting both in-hospital and 28-day mortality, ranging from 15 to 48% in other studies, while the reported specificity is around 90% and higher [13, 19]. The qSOFA has a sensitivity of 47.8% and specificity of 90.5% among sepsis patients who are immunocompromised secondary to hematopoietic cell transplantation [9, 13, 19,20,21]. The sensitivity we found is within the wide range as reported by others. However, it appears lower as compared to the sensitivity of the qSOFA among hematopoietic cell transplantation receivers—on the other hand, the specificity of the qSOFA in our cohort of pharmacologically immunocompromised groups was slightly higher. Although qSOFA is considered more user-friendly, since it requires only three, readily available parameters, clinicians should be aware that identification of immunocompromised patients at high risk of poor outcome is hampered.
In our cohort, NEWS outperformed the qSOFA in predicting in-hospital mortality, both among the whole cohort as well as the studied subgroups. The AUROC to predict in-hospital mortality of the NEWS score was higher than the qSOFA in the whole cohort, as well as among immunocompromised patients and among those using high-dose glucocorticoids. The sensitivity of the NEWS ranged from 65.4% in the whole cohort to 80% in immunocompromised patients and 100% among high-dose glucocorticoid users. The specificity ranged from 73.0 to 74.0%. These findings are in line with previously reported sensitivity of 74.0–87.9% and specificity of 42.1%–90.2% to predict sepsis-related mortality in an immunocompetent population has been reported [9, 10, 20]. When comparing our findings among pharmacologically immunocompromised patients to the accuracy of NEWS among other groups of immunocompromised patients due to hematopoietic cell transplantation, it appears that the sensitivity is higher (hematopoietic cell transplantation: 64.9%), while specificity is similar (hematopoietic cell transplantation: 71.2%) [13]. Further, in kidney transplant recipients the NEWS and SOFA performed best in recipients compared to immunocompetent patients, compared to CRB-65, CURB-65, DS-CRB-65, qSOFA, and PSI (Pneumonia Severity Index) score [22]. In our study, the predictive performance of NEWS was higher than qSOFA in the whole cohort, as well as in immunocompromised patients in general and specifically in patients on high-dose glucocorticoids, in line with observations among patients with a suspected infection after hematopoietic cell transplantation [13].
The 28-day mortality rates in our study were 6.2% for the total cohort and 5.9% among patients who were pharmacologically immunocompromised. It should be noted that the studied population represents a group of patients with a severe infection at risk of developing sepsis and those who already meet sepsis criteria: a clinically relevant group of patients seen daily at the ED. More than half of the patients met Sepsis-3 criteria, while 5.1% was admitted to the ICU and 3.6% died in hospital. The ICU admission rate appears to be lower than in other studies, which could be explained by the relatively low number of patients with sepsis. Another explanation might be the support for more complex care at the general wards by ICU outreach teams in the Netherlands, which lowers the requirement for ICU admission. For the different groups of immunosuppressives, 28-day mortality rates ranged from 0 to 12%, with the highest mortality rate observed in patients on high-dose (> 15 mg/day) glucocorticoids. Although infections are a well-known risk associated with use of immunosuppressive drugs, the risk of deterioration and sepsis development varies between different classes of immunosuppressive drugs due to their different target cell(s) [17, 23,24,25,26]. While some drugs have very specific targets, glucocorticoids have broad effects on the immune system by their anti-inflammatory properties [27]. Previous studies among immunocompromised patients with an infection demonstrated similar or even better outcomes as compared to immunocompetent patients [5, 12, 28,29,30], with 28-day mortality rates from 8 to 32% [5, 12, 31]. Only a few studies took the specific immunosuppressive drug class and/or dose administered into account, rather than considering a general immunocompromised state when using any immunosuppressive drug or having specific co-morbidity (e.g., cancer, history of solid organ transplantation). The relevance of investigating specific immunosuppressive drug classes is supported by our findings, demonstrating an increased in-hospital and 28-day mortality risk among high-dose glucocorticoid users, while use of IMPDH inhibitors was associated with a lower 28-day mortality risk. In a study among rheumatic patients on either conventional/biological disease-modifying anti-rheumatic drugs (cs/b DMARDs) or glucocorticoids who developed sepsis, high-dose glucocorticoids were also associated with mortality, while bDMARD users had a lower risk on mortality [32]. In contrast, however, a study using health care insurance data demonstrated lower in-hospital mortality from sepsis among glucocorticoid users (not stratified to the dose) and among patients taking non-steroid immunosuppressive drugs. Yet, patients taking immunosuppressive drugs were more frequently admitted to the ICU because of sepsis and had a longer length of stay in hospital [29]. Unfortunately, the drug class was not further specified among the non-steroid immunosuppressive drug users.
Strengths and limitations
There were several limitations to our study. First, the diagnosis of sepsis was based upon the combination of a (suspected) infection and a qSOFA/SOFA score of ≥ two according to information present in the electronic patient chart. Patients in our dataset could well be using ≥ one immunosuppressive drug at the same time and interactions and effects of concomitantly used agents cannot be ruled out. We stratified patients into groups based on the immunosuppressive drug use to identify specific drugs that would affect the predictive performance of the qSOFA and NEWS. Consequently, a number of patients on TNF blocker and selective immunosuppressives are small, potentially underpowered, and therefore at risk for type II error. Although we do not have evidence to support that the inclusion time from 8:00 to 23:00 h may have caused selection bias, the risk of such bias should be taken into account when interpreting the evidence provided in the study. It should be noted that there is an inherent risk of overfitting the model that we developed in this single-center cohort. Although the results do allow to expand understanding of the potential risks of immunosuppressives among patients with an infection at the ED, the model should not be used to screen patients without external validation.
Conclusion
We found NEWS to have better discriminative accuracy than qSOFA in predicting in-hospital mortality from sepsis. Patients using more than 15 mg glucocorticoids per day (prednisone equivalent dosage) are particularly at risk for in-hospital mortality. Adjusting the NEWS for high-dose glucocorticoid use does not further improve its predictive performance. As a clinical consequence, we recommend using the NEWS to identify patients with infection at high risk of poor outcome.
Data availability
Data and script are available upon reasonable request.
Change history
26 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s15010-024-02282-1
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–10. https://doi.org/10.1001/jama.2016.0287.
Tolsma V, Schwebel C, Azoulay E, Darmon M, Souweine B, Vesin A, Goldgran-Toledano D, Lugosi M, Jamali S, Cheval C, Adrie C, Kallel H, Descorps-Declere A, Garrouste-Orgeas M, Bouadma L, Timsit JF. Sepsis severe or septic shock: outcome according to immune status and immunodeficiency profile. Chest. 2014;146:1205–13. https://doi.org/10.1378/chest.13-2618.
Jamme M, Daviaud F, Charpentier J, Marin N, Thy M, Hourmant Y, Mira JP, Pène F. Time course of septic shock in immunocompromised and nonimmunocompromised patients. Crit Care Med. 2017;45:2031–9. https://doi.org/10.1097/CCM.0000000000002722.
Joost I, Kaasch A, Pausch C, Peyerl-Hoffmann G, Schneider C, Voll RE, Seifert H, Kern WV, Rieg S. Staphylococcus aureus bacteremia in patients with rheumatoid arthritis—data from the prospective INSTINCT cohort. J Infect. 2017;74:575–84. https://doi.org/10.1016/j.jinf.2017.03.003.
Gotur DB, Masud FN, Ezeana CF, Nisar T, Paranilam J, Chen S, Puppala M, Wong STC, Zimmerman JL. Sepsis outcomes in solid organ transplant recipients. Transpl Infect Dis. 2020;22: e13214. https://doi.org/10.1111/tid.13214.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–11. https://doi.org/10.1016/S0140-6736(19)32989-7.
Gauer R, Forbes D, Boyer N. Sepsis: diagnosis and management. Am Fam Physician. 2020;101:409–18. https://doi.org/10.1371/JOURNAL.PONE.0129305.
Gauer RL. Early recognition and management of sepsis in adults: the first six hours. Am Fam Physician. 2013;88:44–53.
Usman OA, Usman AA, Ward MA. Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the emergency department. Am J Emerg Med. 2019;37:1490–7. https://doi.org/10.1016/j.ajem.2018.10.058.
Brink A, Alsma J, Verdonschot RJCG, Rood PPM, Zietse R, Lingsma HF, Schuit SCE. Predicting mortality in patients with suspected sepsis at the emergency department; a retrospective cohort study comparing qSOFA, SIRS and national early warning score. PLoS ONE. 2019;14: e0211133. https://doi.org/10.1371/journal.pone.0211133.
Kovach CP, Fletcher GS, Rudd KE, Grant RM, Carlbom DJ. Comparative prognostic accuracy of sepsis scores for hospital mortality in adults with suspected infection in non-ICU and ICU at an academic public hospital. PLoS ONE. 2019;14: e0222563. https://doi.org/10.1371/journal.pone.0222563.
Kalil AC, Syed A, Rupp ME, Chambers H, Vargas L, Maskin A, Miles CD, Langnas A, Florescu DF. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin Infect Dis. 2015;60:216–22. https://doi.org/10.1093/cid/ciu789.
Lind ML, Phipps AI, Mooney S, Liu C, Fohner A, Patel K, Ueda M, Pergam SA. Predictive value of 3 clinical criteria for sepsis (quick sequential organ failure assessment, systemic inflammatory response syndrome, and national early warning score) with respect to short-term mortality in allogeneic hematopoietic cell transplant recipients with suspected infections. Clin Infect Dis. 2021;72:1220–9. https://doi.org/10.1093/cid/ciaa214.
Sepsis bij volwassenen in prehospitale en SEH-fase. Nijmegen; 2012. https://www.nvsha.nl/files/620/Richtlijn_Sepsis_-_definitief.pdf. Accessed 21 Jan 2021.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;7: g7594. https://doi.org/10.1136/bmj.g7594.
Gea-Banacloche JC, Opal SM, Jorgensen J, Carcillo JA, Sepkowitz KA, Cordonnier C. Sepsis associated with immunosuppressive medications: an evidence-based review. Crit Care Med. 2004;32:S578–90. https://doi.org/10.1097/01.ccm.0000143020.27340.ff.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;17:77. https://doi.org/10.1186/1471-2105-12-77.
Jiang J, Yang J, Mei J, Jin Y, Lu Y. Head-to-head comparison of qSOFA and SIRS criteria in predicting the mortality of infected patients in the emergency department: a meta-analysis. Scand J Trauma Resusc Emerg Med. 2018;26:56. https://doi.org/10.1186/s13049-018-0527-9.
Goulden R, Hoyle MC, Monis J, Railton D, Riley V, Martin P, Martina R, Nsutebu E. qSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg Med J. 2018;35:345–9. https://doi.org/10.1136/emermed-2017-207120.
Dykes LA, Heintz SJ, Heintz BH, Livorsi DJ, Egge JA, Lund BC. Contrasting qSOFA and SIRS criteria for early sepsis identification in a veteran population. Fed Pract. 2019;36:S21–4.
Müller-Plathe M, Osmanodja B, Barthel G, Budde K, Eckardt KU, Kolditz M, Witzenrath M. Validation of risk scores for prediction of severe pneumonia in kidney transplant recipients hospitalized with community-acquired pneumonia. Infection. 2023. https://doi.org/10.1007/s15010-023-02101-z.
Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, Zhang D, Brunetta PG. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–33. https://doi.org/10.1002/art.27233.
Afzali A, Park CJ, Zhu K, Hu JK, Sharma P, Sinanan MN, Lee SD. Preoperative use of methotrexate and the risk of early postoperative complications in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:1887–95. https://doi.org/10.1097/MIB.0000000000000780.
Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. https://doi.org/10.1056/NEJMoa032534.
Díaz-Lagares C, Pérez-Alvarez R, García-Hernández FJ, Ayala-Gutiérrez MM, Callejas JL, Martínez-Berriotxoa A, Rascón J, Caminal-Montero L, Selva-O’Callaghan A, Oristrell J, Hidalgo C, Gómez-de-la-Torre R, Sáez L, Canora-Lebrato J, Camps MT, Ortego-Centeno N, Castillo-Palma MJ, Ramos-Casals M, BIOGEAS Study Group. Rates of, and risk factors for, severe infections in patients with systemic autoimmune diseases receiving biological agents off-label. Arthritis Res Ther. 2011;13:R112. https://doi.org/10.1186/ar3397.
Marik PE. The role of glucocorticoids as adjunctive treatment for sepsis in the modern era. Lancet Respir Med. 2018;6:793–800. https://doi.org/10.1016/S2213-2600(18)30265-0.
Abramovich E, Barrett O, Dreiher J, Novack V, Abu-Shakra M. Incidence and variables associated with short and long-term mortality in patients with systemic lupus erythematosus and sepsis admitted in intensive care units. Lupus. 2018;27:1936–43. https://doi.org/10.1177/0961203318796288.
Oh S-Y, Cho S, Lee H, Chang EJ, Min SH, Ryu HG. Sepsis in patients receiving immunosuppressive drugs in Korea: analysis of the national insurance database from 2009 to 2013. Acute Crit Care. 2015;30:249–57. https://doi.org/10.4266/KJCCM.2015.30.4.249.
Vaidie J, Peju E, Jandeaux LM, Lesouhaitier M, Lacherade JC, Guillon A, Wittebole X, Asfar P, Evrard B, Daix T, Vignon P, François B. Long-term immunosuppressive treatment is not associated with worse outcome in patients hospitalized in the intensive care unit for septic shock: the PACIFIC study. Crit Care. 2023;27:340. https://doi.org/10.1186/s13054-023-04626-z.
Husabø G, Nilsen RM, Flaatten H, Solligård E, Frich JC, Bondevik GT, Braut GS, Walshe K, Harthug S, Hovlid E. Early diagnosis of sepsis in emergency departments, time to treatment, and association with mortality: an observational study. PLoS ONE. 2020;15: e0227652. https://doi.org/10.1371/journal.pone.0227652.
Richter A, Listing J, Schneider M, Klopsch T, Kapelle A, Kaufmann J, Zink A, Strangfeld A. Impact of treatment with biologic DMARDs on the risk of sepsis or mortality after serious infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1667–73. https://doi.org/10.1136/annrheumdis-2015-207838.
Funding
No funding is received.
Author information
Authors and Affiliations
Contributions
TJO and JCM designed the study. HMEAS, RH and LB performed data collection and statistical analyses. HMEAS and LB drafted the first version of the manuscript. JCM, DFP and HRB supervised the statistical analyses and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boekhoud, L., Schaap, H.M.E.A., Huizinga, R.L. et al. Predictive performance of NEWS and qSOFA in immunocompromised sepsis patients at the emergency department. Infection (2024). https://doi.org/10.1007/s15010-024-02247-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-024-02247-4