Abstract
Background and Objective
Despite the significant burden of Plasmodium falciparum (Pf) malaria and the licensure of two vaccines for use in infants and young children that are partially effective in preventing clinical malaria caused by Pf, a highly effective vaccine against Pf infection is still lacking. Live attenuated vaccines using Pf sporozoites as the immunogen (PfSPZ Vaccines) hold promise for addressing this gap. Here we review the safety and efficacy of two of the most promising PfSPZ approaches: PfSPZ Vaccine (radiation attenuated PfSPZ) and PfSPZ-CVac (chemo-attenuated PfSPZ).
Methods
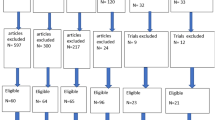
We conducted a systematic review and meta-analysis by searching PubMed, EMBASE, SCOPUS, CENTRAL, and WOS until 22nd December 2021. We included randomized controlled trials (RCTs) of these two vaccine approaches that measured protection against parasitaemia following controlled human malaria infection (CHMI) in malaria-naive and malaria-exposed adults or following exposure to naturally transmitted Pf malaria in African adults and children (primary outcome) and that also measured the incidence of solicited and unsolicited adverse events as indicators of safety and tolerability after vaccination (secondary outcome).
We included randomized controlled trials (RCTs) that measured the detected parasitaemia after vaccination (primary outcome) and the incidence of various solicited and unsolicited adverse events (secondary outcome).
The quality of the included RCTs using the Cochrane ROB 1 tool and the quality of evidence using the GRADE system were evaluated. We pooled dichotomous data using the risk ratio (RR) for development of parasitemia in vaccinees relative to controls as a measure of vaccine efficacy (VE), including the corresponding confidence interval (CI). This study was registered with PROSPERO (CRD42022308057).
Results
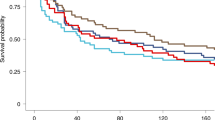
We included 19 RCTs. Pooled RR favoured PfSPZ Vaccine (RR: 0.65 with 95% CI [0.53, 0.79], P = 0.0001) and PfSPZ-table (RR: 0.42 with 95% CI [0.27, 0.67], P = 0.0002) for preventing parasitaemia, relative to normal saline placebo. Pooled RR showed no difference between PfSPZ Vaccine and the control in the occurrence of any solicited adverse event (RR: 1.00 with 95% CI [0.82, 1.23], P = 0.98), any local solicited adverse events (RR: 0.73 with 95% CI [0.49, 1.08], P = 0.11), any systemic solicited adverse events (RR: 0.94 with 95% CI [0.75, 1.17], P = 0.58), and any unsolicited adverse event (RR: 0.93 with 95% CI [0.78, 1.10], P = 0.37).
Conclusion
PfSPZ and PfSPZ-CVacs showed comparable efficacy. Therefore, they can introduce a promising strategy for malaria prophylaxis, but more large-scale field trials are required to sustain efficacy and yield clinically applicable findings.





Similar content being viewed by others
Data availability
The data are available on request.
References
Malaria vaccines: preferred product characteristics and clinical development considerations. https://www.who.int/publications-detail-redirect/9789240057463. Accessed 10 Nov 2023.
Autoridad Nacional del Servicio Civil. World malaria report 2021. Angewandte Chemie International Edition, 6(11), 951–952. 2021;2013–5.
World Health Organization (WHO). Malaria. Geneva: World Health Organization; 2021.
WHO Global. World malaria report 2019. Brazzaville: WHO Regional Office for Africa; 2019. p. 1–232.
Sulyok Z, Fendel R, Eder B, Lorenz FR, Kc N, Karnahl M, et al. Heterologous protection against malaria by a simple chemoattenuated PfSPZ vaccine regimen in a randomized trial. Nat Commun. 2021;12:1–10.
Zheng J, Pan H, Gu Y, Zuo X, Ran N, Yuan Y, et al. Prospects for malaria vaccines: pre-erythrocytic stages, blood stages, and transmission-blocking stages. BioMed Res Int. 2019;2019:1.
World Health Organization. Global technical strategy for malaria 2016–2030, 2021 update. Geneva: World Health Organization; 2021. p. 1–40.
Choumet V, Attout T, Chartier L, Khun H, Sautereau J, Robbe-Vincent A, et al. Visualizing non infectious and infectious anopheles gambiae blood feedings in naive and saliva-immunized mice. PLoS ONE. 2012;7:e50464. https://doi.org/10.1371/journal.pone.0050464.
Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Vaccine. 2015;33:D13-23.
Duffy PE, Sahu T, Akue A, Milman N, Anderson C. Pre-erythrocytic malaria vaccines: identifying the targets. Expert Rev Vaccines. 2012;11:1261–80.
Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542:445–9.
Lyke KE, Ishizuka AS, Berry AA, Chakravarty S, DeZure A, Enama ME, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci USA. 2017;114:2711–6.
Spring M, Polhemus M, Ockenhouse C. Controlled human malaria infection. J Infect Dis. 2014;209:S40–5.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583.
Innovation VH. Covidence systematic review software. Melbourne, Australia.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. Rating quality of evidence and strength of recommendations: what is “quality of evidence” and why is it important to clinicians? BMJ Br Med J. 2008;336:995.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. Rating quality of evidence and strength of recommendations: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ Br Med J. 2008;336:924.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ Br Med J. 2003;327:557.
RevMan | Cochrane Training. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. Accessed 3 Aug 2021.
MedCalc® Statistical Software version 20.027 (MedCalc Software Ltd, Ostend, Belgium. 2022. https://www.medcalc.org
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–60.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17:498–509.
Jongo SA, Preston Church LW, Mtoro AT, Schindler T, Chakravarty S, Ruben AJ, et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ vaccine in Tanzanian Adults. Clin Infect Dis. 2020;71:2849–57.
Steinhardt LC, Richie TL, Yego R, Akach D, Hamel MJ, Gutman JR, et al. Safety, tolerability, and immunogenicity of plasmodium falciparum sporozoite vaccine administered by direct venous inoculation to infants and young children: Findings from an age de-escalation, dose-escalation, double-blind, randomized controlled study in. Clin Infect Dis. 2020;71:1063–71.
Jongo SA, Church LWP, Mtoro AT, Chakravarty S, Ruben AJ, Swanson PA, et al. Safety and differential antibody and T-cell responses to the plasmodium falciparum sporozoite malaria vaccine, PfSPZ vaccine, by age in tanzanian adults, adolescents, children, and infants. Am J Trop Med Hyg. 2019;100:1433–44.
Olotu A, Urbano V, Hamad A, Eka M, Chemba M, Nyakarungu E, et al. Advancing global health through development and clinical trials partnerships: a randomized, placebo-controlled, double-blind assessment of safety, tolerability, and immunogenicity of pfspz vaccine for malaria in healthy equatoguinean men. Am J Trop Med Hyg. 2018;98:308–18.
Jongo SA, Shekalaghe SA, Preston Church LW, Ruben AJ, Schindler T, Zenklusen I, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg. 2018;99:338–49.
Sissoko MS, Healy SA, Katile A, Zaidi I, Hu Z, Kamate B, et al. Safety and efficacy of a three-dose regimen of Plasmodium falciparum sporozoite vaccine in adults during an intense malaria transmission season in Mali: a randomised, controlled phase 1 trial. Lancet Infect Dis. 2022;22:377–89.
Jongo SA, Urbano V, Preston Church LW, Olotu A, Manock SR, Schindler T, et al. Immunogenicity and protective efficacy of radiation-attenuated and chemo-attenuated PfSPZ vaccines in equatoguinean adults. Am J Trop Med Hyg. 2021;104:283–93.
Murphy SC, Deye GA, Kim Lee Sim B, Galbiati S, Kennedy JK, Cohen KW, et al. PfSPZ-CVac efficacy against malaria increases from 0% to 75% when administered in the absence of erythrocyte stage parasitemia: a randomized, placebo-controlled trial with controlled human malaria infection. PLoS Pathog. 2021;17:1–23.
Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight. 2017;2:1–14.
Mwakingwe-Omari A, Healy SA, Lane J, Cook DM, Kalhori S, Wyatt C, et al. Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature. 2021;595:289–94.
Lyke KE, Singer A, Berry AA, Reyes S, Chakravarty S, James ER, et al. Multidose priming and delayed boosting improve Plasmodium falciparum Sporozoite vaccine efficacy against heterologous P. falciparum controlled human malaria infection. Clin Infect Dis. 2021;73:e2424–35.
Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–65.
Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 2016;22:614–23.
Oneko M, Steinhardt LC, Yego R, Wiegand RE, Swanson PA, Kc N, et al. Safety, immunogenicity and efficacy of PfSPZ Vaccine against malaria in infants in western Kenya: a double-blind, randomized, placebo-controlled phase 2 trial. Nat Med. 2021;27:1636–45.
Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James ER, et al. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2014;91:471–80.
Kamau E, Alemayehu S, Feghali KC, Saunders D, Ockenhouse CF. Multiplex qPCR for detection and absolute quantification of malaria. PLoS ONE. 2013;8:e71539.
Goh YS, McGuire D, Rénia L. Vaccination with sporozoites: models and correlates of protection. Front Immunol. 2019;10:1227.
EMA. First malaria vaccine receives positive scientific opinion from EMA. European Medicines Agency. 2018. https://www.ema.europa.eu/en/news/first-malaria-vaccine-receives-positive-scientific-opinion-ema. Accessed 10 Nov 2023.
Wagle MV, Marchingo JM, Howitt J, Tan SS, Goodnow CC, Parish IA. The ubiquitin ligase adaptor NDFIP1 selectively enforces a CD8+ T cell tolerance checkpoint to high-dose antigen. Cell Rep. 2018;24:577–84.
Cornberg M, Kenney LL, Chen AT, Waggoner SN, Kim SK, Dienes HP, et al. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front Immunol. 2013. https://doi.org/10.3389/fimmu.2013.00475.
Han S, Asoyan A, Rabenstein H, Nakano N, Obst R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc Natl Acad Sci USA. 2010;107:20453–8.
Michallet M-C, Saltel F, Flacher M, Revillard J-P, Genestier L. Cathepsin-dependent apoptosis triggered by Supraoptimal activation of T lymphocytes: a possible mechanism of high dose tolerance. J Immunol. 2004;172:5405–14.
Haneda K, Sano K, Tamura G, Shirota H, Ohkawara Y, Sato T, et al. Transforming growth factor-β secreted from CD4+ T cells ameliorates antigen-induced eosinophilic inflammation: a novel high-dose tolerance in the trachea. Am J Respir Cell Mol Biol. 1999;21:268–74.
Critchfield JM, Zúñiga-Pflücker JC, Lenardo MJ. Parameters controlling the programmed death of mature mouse T lymphocytes in high-dose suppression. Cell Immunol. 1995;160:71–8.
Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Am J Prev Med. 2015;49:S319–33.
Richie TL, Billingsley PF, Sim BKL, James ER, Chakravarty S, Epstein JE, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33:7452–61.
Jongo SA, Urbano Nsue Ndong Nchama V, Church LWP, Olotu A, Manock SR, Schindler T, et al. Safety and immunogenicity of radiation-attenuated PfSPZ vaccine in equatoguinean infants, children, and adults. Am J Trop Med Hyg. 2023;109:138–46.
Sirima SB, Ouédraogo A, Tiono AB, Kaboré JM, Bougouma EC, Ouattara MS, et al. A randomized controlled trial showing safety and efficacy of a whole sporozoite vaccine against endemic malaria. Sci Transl Med. 2022;14:abj3776. https://doi.org/10.1126/scitranslmed.abj3776.
Garcia CR, Manzi F, Tediosi F, Hoffman SL, James ER. Comparative cost models of a liquid nitrogen vapor phase (LNVP) cold chain-distributed cryopreserved malaria vaccine vs. a conventional vaccine. Vaccine. 2013;31:380–6.
Coulibaly D, Kone AK, Traore K, Niangaly A, Kouriba B, Arama C, et al. PfSPZ-CVac malaria vaccine demonstrates safety among malaria-experienced adults: a randomized, controlled phase 1 trial. EClinicalMedicine. 2022;52:101579.
Jongo SA, Church LWP, Nchama VUNN, Hamad A, Chuquiyauri R, Kassim KR, et al. Multi-dose priming regimens of PfSPZ vaccine: safety and efficacy against controlled human malaria infection in Equatoguinean adults. Am J Trop Med Hyg. 2022;106:1215–26.
Mordmüller B, Sulyok Z, Sulyok M, Molnar Z, Lalremruata A, Calle CL, et al. A PfSPZ vaccine immunization regimen equally protective against homologous and heterologous controlled human malaria infection. NPJ Vaccines. 2022;7:100.
Acknowledgements
Mohamed Abouzid is a participant in the STER Internationalization of Doctoral Schools Program of the NAWA Polish National Agency for Academic Exchange No. PPI/STE/2020/1/00014/DEC/02.
Funding
We received no funding for this study.
Author information
Authors and Affiliations
Contributions
MTA conceived and designed the work. BA and MTA collected the data. MTA, MAE, MG, MAgheit, HB, and MAzid gave substantial contributions to data acquisition and interpretation for the work; MTA, MKA, and HB drafted the manuscript. BA, SA, MTA, MAE, and MAzid revised it critically for important intellectual content. All the authors gave the final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abuelazm, M.T., Elzeftawy, M.A., Kamal, M.A. et al. Protective efficacy and safety of radiation-attenuated and chemo-attenuated Plasmodium Falciparum sporozoite vaccines against controlled and natural malaria infection: a systematic review and meta-analysis of randomized controlled trials. Infection (2024). https://doi.org/10.1007/s15010-024-02174-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s15010-024-02174-4